MC4R KO Mouse Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of advanced preclinical models for NASH, providing comprehensive services to evaluate drug efficacy and accelerate the development of novel treatments. These models enable detailed studies of disease mechanisms and help in the assessment of therapeutic interventions.
Introduction
Non-alcoholic steatohepatitis (NASH) is a progressive liver disease that occurs in individuals without significant alcohol consumption. It is characterized by the accumulation of fat in the liver (steatosis), inflammation, and hepatocellular injury, which can progress to fibrosis, cirrhosis, and ultimately liver failure. NASH is a major concern worldwide, as it is commonly associated with obesity, type 2 diabetes, and metabolic syndrome. The condition is becoming more prevalent due to the rising global incidence of these metabolic diseases. NASH can be challenging to diagnose early, as it often develops silently over many years. In the absence of effective treatments, early detection and intervention are crucial in preventing disease progression. Due to the complexity and varied pathophysiology of NASH, research into novel therapeutic strategies is of utmost importance. Animal models play a critical role in understanding the mechanisms of the disease and in testing potential drugs.
Disease Models and Applications
The MC4R knockout (KO) mouse model is a genetically engineered model used to study the role of the melanocortin-4 receptor (MC4R) in metabolic disorders. The model is constructed by disrupting the MC4R gene, leading to obesity, insulin resistance, and other metabolic syndromes. This model mimics key aspects of human metabolic diseases like obesity, type 2 diabetes, and NASH. A significant feature of this model is its ability to develop spontaneous obesity and associated liver steatosis. The MC4R KO mouse model is highly valuable for studying the mechanisms underlying metabolic diseases and testing potential therapeutic agents. However, a limitation is that the model may not fully replicate the human condition of NASH due to differences in underlying metabolic pathways. Additionally, the obesity in MC4R KO mice can be severe, potentially overshadowing subtle metabolic disease phenotypes.
- Simulates: The MC4R KO mouse model simulates metabolic diseases such as obesity, insulin resistance, type 2 diabetes, and associated complications like NASH.
- Evaluates Drugs: The model is useful for evaluating drugs targeting obesity, insulin resistance, glucose metabolism, and anti-inflammatory treatments. It allows for the testing of drug candidates for improving metabolic dysfunction and reversing liver steatosis.
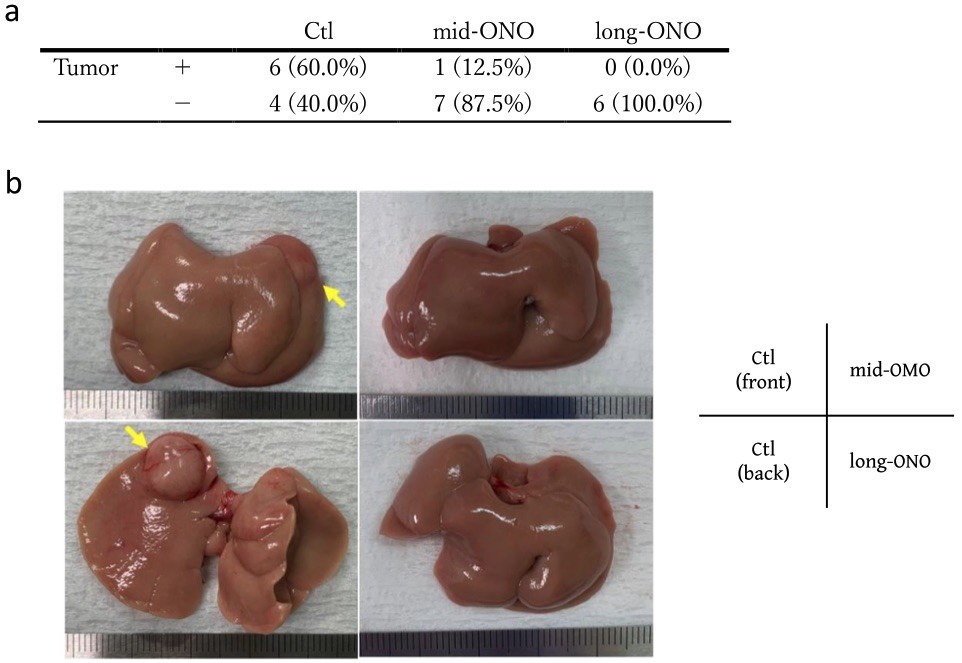 Fig. 1 Evaluation of liver tumor occurrence in Mc4r-KO NASH model mice at 36 weeks of age.1
Fig. 1 Evaluation of liver tumor occurrence in Mc4r-KO NASH model mice at 36 weeks of age.1
Measurements
We offer a variety of measurements for evaluating drug efficacy in rodent models of metabolic diseases, including the MC4R KO Mouse Model. Our comprehensive approach involves advanced technologies, such as:
- General observations: body weight, food intake, activity levels, and liver weight.
- Histopathology: liver tissue analysis using H&E staining to observe steatosis, inflammation, and fibrosis.
- Immunohistochemistry: Detection of immune cell infiltration in the liver (e.g., macrophages, T-cells).
- Cytokine profiling (e.g., ELISA): Quantifying inflammatory mediators like TNF-α, IL-6, IL-1β.
- Hematology analysis and serum biomarkers: Analysis of liver enzymes (e.g., ALT, AST), cholesterol, triglycerides, and other biomarkers of liver function.
- Gene/protein expression profiling: RT qPCR and Western blot for assessing the expression of genes related to lipid metabolism, insulin signaling, and fibrosis markers.
In addition to standard tests, our expert team provides personalized consultation for experimental design, model selection, and data analysis, ensuring a tailored approach to each research project.
Related Services
In addition to the MC4R KO mouse model, we offer other models for studying NASH, including diet induced models and models induced by chemicals such as CCl4. These models enable a broader understanding of the disease and its therapeutic options.
- Diet induced Obesity (DIO) Mouse NASH Model
- High-Fat Diet induced NASH Model
- Methionine Choline-Deficient (MCD) Diet induced NASH Model
- Choline-Deficient L-Amino Acid-Defined (CDAA) Diet induced NASH Model
- High-Fat & High-Carbohydrate Diet induced NASH Model
- High-Fat & High-Cholesterol Diet induced NASH Model
- High-Fat & High-Cholesterol Diet & Fructose induced NASH Model
- High-Fat & Fructose induced NASH Model
- Diethylnitrosamine (DEN) & High-Fat & High-Carbohydrate Diet induced NASH Model
- High-Fat & CCL4 induced NASH Model
- Streptozotocin (STZ) & High-Fat induced NASH Model
- LDLR KO Mouse Model
Advantages
- Expertise in Preclinical Research: We have extensive experience in developing and utilizing advanced animal models for a variety of diseases, including NASH, ensuring high-quality results and robust data.
- Tailored Solutions: We offer customized research services based on your specific project needs, providing personalized experimental designs and flexible models.
- State-of-the-Art Technology: Our labs are equipped with the latest diagnostic and analytical tools, enabling precise measurement and high-resolution data collection.
- Comprehensive Support: From model selection to data analysis, we provide full-service support throughout your project, ensuring seamless integration of all stages of your research.
- Validated Models: We provide well-established, reliable, and validated animal models that have been proven to accurately replicate human disease conditions.
- Timely Delivery: We understand the importance of timely results in drug development and offer efficient project management to ensure deadlines are met.
- Global Reach: Our services are trusted by clients around the world, enabling broad access to cutting-edge research capabilities for companies and institutions.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What is NASH, and why is it important to study?
A: Non-alcoholic steatohepatitis (NASH) is a severe form of non-alcoholic fatty liver disease (NAFLD), characterized by liver inflammation and damage. Understanding NASH is crucial for developing effective treatments, as it can progress to cirrhosis and liver cancer.
-
Q: What types of animal models do you offer for NASH research?
A: We provide a variety of preclinical models for NASH, including diet induced models, genetic knockout models like the MC4R KO mice, and chemical induced models, each suited to different research objectives.
-
Q: How can your models help in evaluating NASH treatments?
A: Our models allow for the evaluation of novel therapeutic candidates by simulating key aspects of NASH pathogenesis, including liver steatosis, inflammation, and fibrosis. These models are invaluable in assessing drug efficacy and the mechanism of action.
-
Q: What measurements are used to assess drug efficacy in NASH models?
A: We offer a wide range of measurements, including histopathological analysis, liver enzyme levels, cytokine profiling, and gene expression analysis to assess the impact of therapeutic interventions.
-
Q: Can you help with experimental design and data analysis?
A: Yes, our expert team is available to assist with experimental design, model selection, and data interpretation to ensure the success of your research project.
Published Data
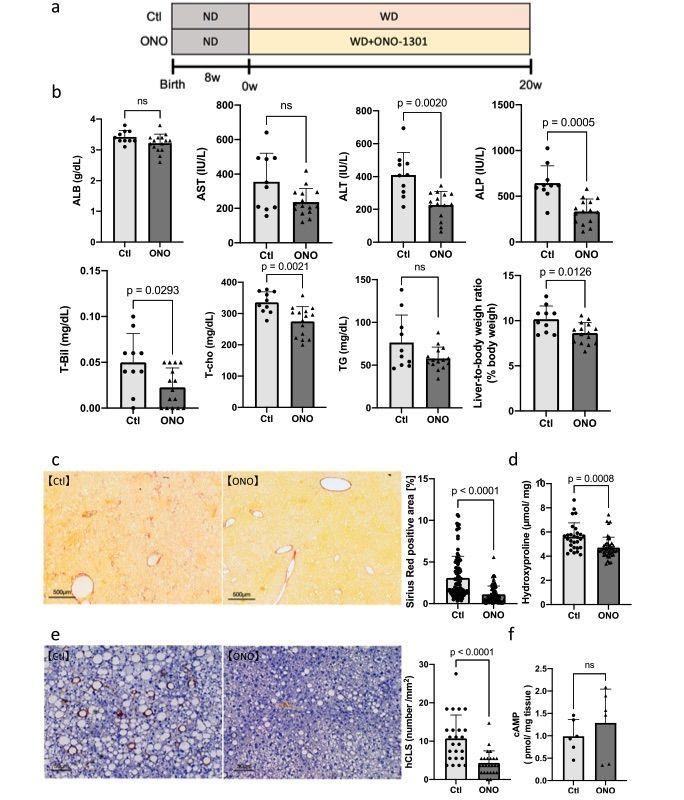 Fig. 2 Therapeutic effects of ONO-1301 (20 weeks of treatment) in the Mc4r-KO NASH model mice.1
Fig. 2 Therapeutic effects of ONO-1301 (20 weeks of treatment) in the Mc4r-KO NASH model mice.1
To evaluate whether the therapeutic effect of ONO-1301 varied depending on the status of NASH, we divided the mice into three groups: the Ctl group, which was fed a normal diet (ND) until 8 weeks and then a western diet (WD) until 28 weeks; the Mid-ONO group, which was fed ND until 8 weeks, followed by 20 weeks of WD, and then WD with ONO-1301 for 8 weeks; and the Long-ONO group, which was fed ND until 8 weeks, followed by WD with ONO-1301 for 28 weeks (Fig. 2a). In the Mid-ONO group, serum levels of ALB, AST, T-cho, and TG did not show significant changes compared to the Ctl group. However, serum levels of ALT and ALP were significantly reduced in the Mid-ONO group (ALT: 234.5 ± 95.7 IU/L, p = 0.0052; ALP: 367.1 ± 116.0 IU/L, p = 0.0061), and the liver-to-body weight ratio was also significantly lower (9.64 ± 1.38 g, p = 0.0399) compared to the Ctl group (Fig. 2b). These results suggest that ONO-1301 has a therapeutic effect in the Mid-ONO group, and long-term use (Long-ONO group) showed sustained improvements in lipid-related markers. The evaluation of fibrosis revealed that the Sirius Red-stained area and hydroxyproline levels were significantly reduced in the ONO-1301 groups compared to the control group (Sirius Red: Mid-ONO group: 3.09% ± 2.59%, Long-ONO group: 2.65% ± 2.27%, p < 0.0001; Hydroxyproline: Mid-ONO group: 4.94 ± 1.79 nmol/mg, Long-ONO group: 5.45 ± 2.94 nmol/mg, p = 0.0069; Fig. 2c, 2d). These findings confirm the sustained therapeutic effects of ONO-1301 in improving both lipid markers and fibrosis.
Reference
- Motegi, Satoko et al. "A novel prostaglandin I2 agonist, ONO-1301, attenuates liver inflammation and suppresses fibrosis in non-alcoholic steatohepatitis model mice." Inflammation and Regeneration vol. 42,1 3. 1 Feb. 2022, DOI:10.1186/s41232-021-00191-6. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
