Pacemaker induced Atrial Fibrillation Modeling & Pharmacodynamics Service
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, characterized by irregular and rapid heartbeats originating from the atria. This chaotic electrical activity leads to inefficient blood pumping, significantly increasing the risk of stroke, heart failure, and reduced quality of life for millions worldwide. Understanding its complex pathophysiology is crucial for developing effective treatments.
Creative Biolabs is dedicated to advancing cardiovascular research, offering a variety of well-established, highly translational models to evaluate the efficacy of novel antiarrhythmic compounds and device strategies.
Pacemaker-Induced AF Model
The pacemaker-induced AF model is a highly relevant preclinical tool that leverages the clinical observation of increased AF incidence in patients with implanted pacemakers. This model serves as a robust platform for investigating the mechanisms underlying AF initiation and progression, as well as for evaluating potential therapeutic interventions. By mimicking the chronic, non-physiological electrical stimulation that contributes to AF in humans, this model allows for the controlled induction and maintenance of a stable AF substrate, enabling comprehensive studies into electrical, structural, and autonomic remodeling.
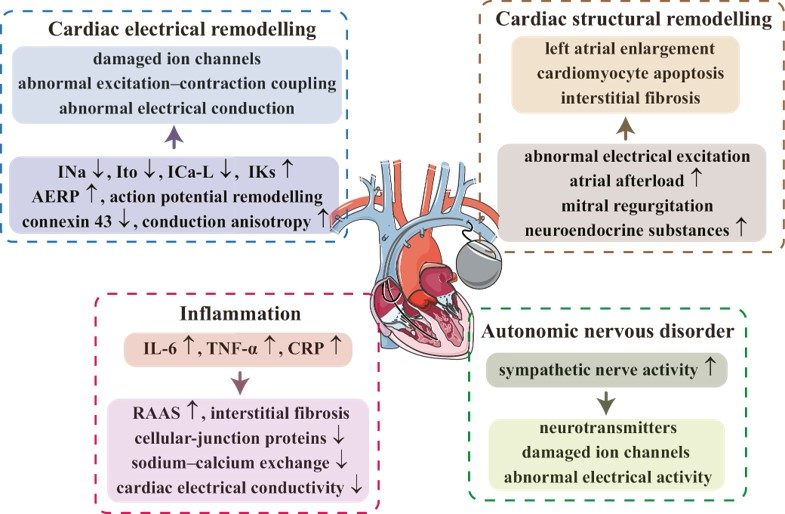 Fig.1 Mechanism of pacemaker implantation-induced AF.1
Fig.1 Mechanism of pacemaker implantation-induced AF.1
Model Construction Steps
The construction of the pacemaker-induced AF model primarily involves chronic, long-term atrial pacing to induce a pro-arrhythmic substrate.
01Surgical Implantation
A pacing electrode is surgically implanted into the right atrium of the rodent (e.g., rat) heart, often via a transesophageal approach for stability and minimal invasiveness.
02External Pacemaker Connection
The electrode is then connected to an external pacemaker device, which can be programmed to deliver continuous or intermittent rapid atrial pacing (RAP).
03Chronic Pacing Protocol
Animals undergo a period of sustained RAP, typically over several days to weeks. This chronic stimulation is crucial for inducing the necessary electrical and structural remodeling in the atria.
04AF Induction and Monitoring
After the pacing period, AF inducibility is tested using burst pacing protocols, and spontaneous AF episodes are monitored using continuous ECG recordings or implantable telemetry systems.
05Validation
The model is validated by confirming the presence of sustained AF episodes and characterizing the associated electrophysiological, structural, and molecular changes.
Strengths and Limitations
Strengths:
- High Clinical Relevance: Directly mirrors a common clinical scenario of pacing-induced AF, enhancing translational potential.
- Reproducible AF Induction: Provides a consistent and reliable method for inducing stable, long-lasting AF episodes.
- Controlled Environment: Allows for precise control over pacing parameters, facilitating mechanistic studies.
- Comprehensive Remodeling: Enables investigation into electrical, structural, and autonomic remodeling processes.
Limitations:
- Invasive Procedure: Requires surgical implantation, which can be a limiting factor in some studies.
- Species Differences: While highly translational, inherent physiological differences between rodents and humans must be considered.
- Specific AF Phenotype: Primarily models pacing-induced AF, which may not encompass all forms of human AF.
Evaluation Platform
Creative Biolabs offers a comprehensive suite of advanced instruments and assays to thoroughly evaluate the effects of interventions within the pacemaker-induced AF model. Our capabilities span biochemical, molecular, cellular, histopathological, behavioral, and imaging analyses, providing a holistic view of disease progression and therapeutic impact.
Key Test Indicators:
- Electrophysiological Parameters: Atrial effective refractory period (AERP), conduction velocity, AF inducibility, AF duration, AF burden, P-wave duration, heart rate variability (HRV).
- Structural Remodeling Markers: Atrial enlargement (echocardiography), fibrosis (histology, collagen assays), myocyte hypertrophy.
- Molecular Markers: Gene expression (e.g., ion channels, fibrosis markers like TGF-beta, inflammatory cytokines like IL-6, microRNAs like MIR-26, MIR-101, Hcn4, Pitx2), protein expression.
- Cellular Function: Calcium handling, ion channel currents (patch clamp).
- Hemodynamic Assessment: Cardiac output, atrial pressure.
Applications
- Simulating Diseases: Primarily used to simulate chronic AF and its associated electrical, structural, and autonomic remodeling. It also provides insights into heart failure-related AF.
- Evaluating Drugs: Ideal for screening and evaluating the efficacy of novel antiarrhythmic drugs, including those targeting ion channels, fibrosis, inflammation, or autonomic nervous system modulation.
- Assessing Treatments: Used to optimize pacing strategies (e.g., different pacing sites, algorithms) and evaluate the impact of other non-pharmacological interventions, such as ablation techniques.
- Mechanistic Studies: Provides a platform to unravel the fundamental molecular and cellular mechanisms driving AF initiation and perpetuation.
Related Atrial Fibrillation Models
Our Advantages
- Years of Experience: Deep scientific knowledge and extensive experience in cardiovascular disease models.
- Customized Study Design: Tailored protocols to precisely meet your unique research objectives.
- Robust & Reproducible Data: Meticulous execution and rigorous quality control for reliable and actionable results.
- State-of-the-Art Facilities: Access to advanced instrumentation and a comprehensive evaluation platform.
- Translational Insights: Expert interpretation of preclinical data to guide successful clinical translation.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is your trusted partner in advancing AF research. Our expertise in the pacemaker-induced AF model and comprehensive service offerings can significantly accelerate your therapeutic development. Contact us today to discuss your project needs.
FAQs
-
Q1: Can this model differentiate between different types of AF, such as paroxysmal versus persistent?
A: While the primary induction typically leads to a more sustained form of AF, the model can be adapted to study aspects of AF progression. By varying pacing parameters and monitoring duration, researchers can observe the transition from more inducible to spontaneously occurring and persistent forms of arrhythmia, offering insights into the mechanisms driving AF chronification.
-
Q2: What specific electrophysiological parameters can be measured using this model?
A: Our evaluation platform allows for a wide array of electrophysiological measurements. These include, but are not limited to, AERP, conduction velocity, AF inducibility thresholds, and the duration and burden of AF episodes. We also assess P-wave morphology and duration, and perform HRV analysis to evaluate autonomic nervous system involvement.
-
Q3: Is it possible to evaluate novel drug targets that address atrial remodeling in this model?
A: Absolutely. The Pacemaker-Induced AF Model is an excellent platform for this purpose. Since chronic pacing induces significant electrical, structural (e.g., fibrosis, atrial enlargement), and autonomic remodeling, it provides a relevant substrate to test compounds designed to reverse or prevent these changes, thereby offering a comprehensive assessment of novel antiarrhythmic strategies.
-
Q4: What animal species are typically used for this model at Creative Biolabs?
A: At Creative Biolabs, we primarily utilize rodent models, particularly rats, for the Pacemaker-Induced AF Model. These species offer advantages in terms of cost-effectiveness, ease of handling, and established genetic manipulation tools. While there are species-specific differences, the fundamental mechanisms of pacing-induced remodeling and AF development show significant translational similarities.
-
Q5: Can this model be combined with other disease models, such as heart failure or hypertension?
A: Yes, the Pacemaker-Induced AF Model can be integrated with other cardiovascular disease models to investigate AF in a more complex pathophysiological context. For instance, combining it with models of heart failure or hypertension can provide valuable insights into how these co-morbidities influence AF development and progression, and how new therapies might perform in such complex patient populations.
Published Data
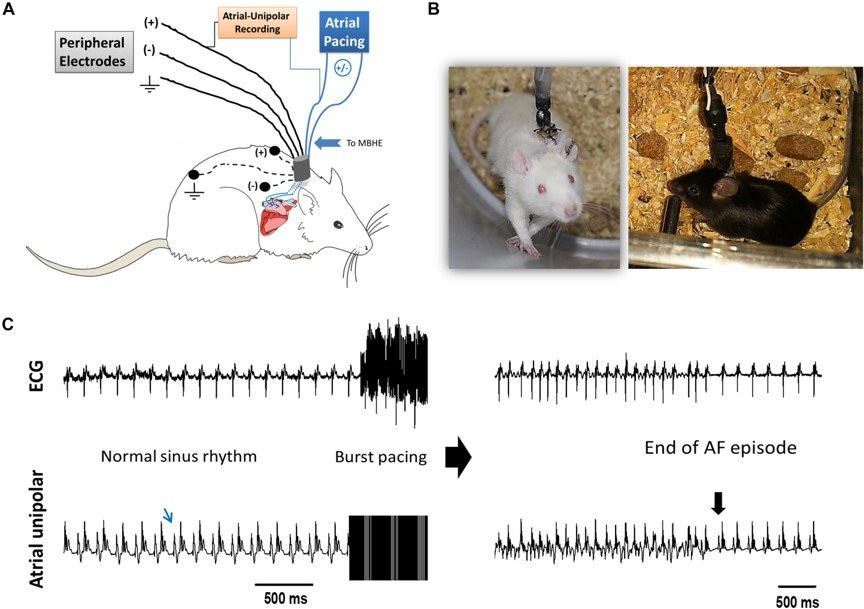 Fig.2 The experimental setup and AF substrate analysis.2
Fig.2 The experimental setup and AF substrate analysis.2
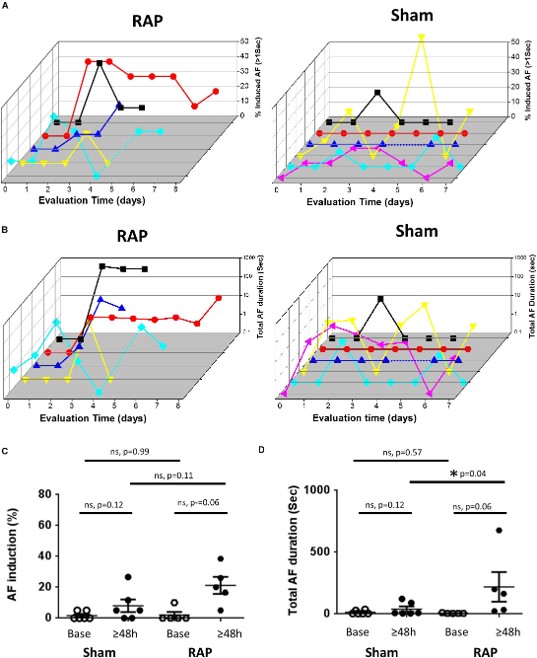 Fig.3 Increased AF substrate in rats exposed to RAP.2
Fig.3 Increased AF substrate in rats exposed to RAP.2
This representative study demonstrated that chronic RAP in rats successfully promotes atrial tissue remodeling and the formation of an AF-prone substrate. The research revealed significant changes in gene expression, including those related to carbohydrate metabolism, TGF-beta, and IL-6 signaling, highlighting the model's utility in uncovering molecular mechanisms relevant to human AF. This work reinforces the translational value of rodent models in AF research.
References
- Wu, Yeshun et al. "Review of the epidemiology, pathogenesis and prevention of atrial fibrillation after pacemaker implantation." Advances in clinical and experimental medicine : official organ Wroclaw Medical University vol. 32,6 (2023): 707-718. Distributed under Open Access license CC BY 3.0, without modification. DOI: 10.17219/acem/157239
- Mulla, Wesam et al. "Rapid Atrial Pacing Promotes Atrial Fibrillation Substrate in Unanesthetized Instrumented Rats." Frontiers in physiology vol. 10 1218. 20 Sep. 2019. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fphys.2019.01218
For Research Use Only.
