High-Fat Diet (HFD) induced Non-Alcoholic Steatohepatitis (NASH) Modeling & Pharmacodynamics Service
Creative Biolabs provides a range of well-established models to evaluate the efficacy of drugs targeting NASH, enabling comprehensive preclinical assessments of potential therapies for liver disease.
Introduction
Non-alcoholic steatohepatitis (NASH) is a severe form of non-alcoholic fatty liver disease (NAFLD) characterized by the accumulation of fat in the liver, inflammation, and varying degrees of liver injury. Unlike alcoholic liver disease, NASH occurs in individuals who do not consume excessive alcohol but are often affected by metabolic conditions such as obesity, type 2 diabetes, and hyperlipidemia. The pathogenesis of NASH involves a complex interplay of factors, including oxidative stress, mitochondrial dysfunction, lipotoxicity, and immune cell activation, leading to inflammation, liver cell damage, and fibrosis. If left untreated, NASH can progress to cirrhosis, liver failure, and liver cancer, making it a major cause of morbidity and mortality worldwide. As the prevalence of obesity and metabolic syndrome increases, NASH has become a growing public health concern. Early diagnosis and intervention are crucial for preventing the progression of the disease to more severe liver conditions. Current treatment strategies are focused on managing the underlying metabolic risk factors, reducing liver inflammation, and preventing fibrosis.
Disease Models and Applications
The High-Fat Diet induced NASH Model is commonly used to replicate the characteristics of human NASH in rodents. This model typically involves feeding rodents a high-fat diet (HFD) for several weeks or months, leading to the development of hepatic steatosis and liver injury. As the disease progresses, hepatic inflammation and fibrosis may also develop, mimicking the stages of NASH in humans. The key advantage of this model is that it closely mirrors the metabolic and histopathological features of human NASH, including fat accumulation in hepatocytes, increased liver enzymes, and inflammation. However, the model has some limitations, such as the fact that it does not always replicate the full range of NASH pathology seen in human patients, particularly with regard to the severity of fibrosis. Despite these limitations, the HFD induced NASH model remains an important tool for preclinical testing of potential therapeutics aimed at treating NASH and related liver diseases.
- Simulates: The High-Fat Diet induced NASH Model simulates non-alcoholic steatohepatitis in rodents, encompassing the key features of hepatic steatosis, inflammation, and liver injury that are commonly observed in human NASH patients.
- Evaluates Drugs: This model is used to evaluate a wide range of therapeutic agents targeting NASH, including those aimed at reducing liver fat accumulation, inflammation, oxidative stress, and fibrosis. It also helps assess the effectiveness of drugs that may slow the progression from NASH to cirrhosis or liver cancer. Common drug classes tested include anti-inflammatory agents, antioxidants, lipid-lowering drugs, and antifibrotic therapies.
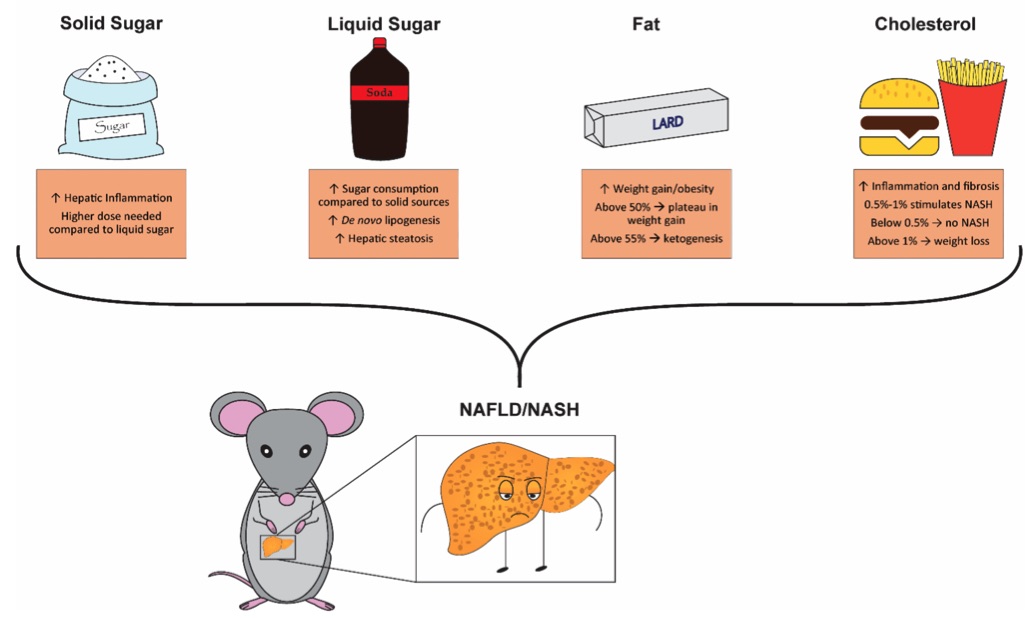 Fig. 1 Summary of key dietary components contributions when modeling non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) in rodents. 1
Fig. 1 Summary of key dietary components contributions when modeling non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) in rodents. 1
Measurements
We offer a variety of measurements for evaluating drug efficacy in the High-Fat Diet induced NASH Model, utilizing an array of advanced technologies, including but not limited to:
- General observations: body weight, liver weight, liver enzyme levels (e.g., ALT, AST), and serum markers for liver function (e.g., bilirubin, albumin).
- Histopathology: Liver tissue examination for steatosis, inflammatory cell infiltration, and fibrosis.
- Immunohistochemistry: Staining for markers of inflammation (e.g., TNF-α, IL-6) and fibrosis (e.g., collagen I, α-SMA) in liver tissues.
- Cytokine profiling (e.g., ELISA): Expression levels of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, which are implicated in the pathogenesis of NASH.
- Gene/protein expression profiling: RT-qPCR and Western blot techniques to measure expression of genes related to lipid metabolism, inflammation, and fibrosis.
- Liver histology scoring: Using standard grading systems like the NAS (Non-Alcoholic Fatty Liver Disease Activity Score) for assessing the degree of steatosis, inflammation, and fibrosis.
Additionally, our scientific team can help design experiments, select models, and analyze data to ensure optimal outcomes for your NASH-related research.
Related Services
In addition to the High-Fat Diet induced NASH Model, we offer alternative methods for inducing NASH in animal models. These models each offer unique insights into the disease process and are useful for testing different therapeutic strategies.
- Diet induced Obesity (DIO) Mouse NASH Model
- Methionine Choline-Deficient (MCD) Diet induced NASH Model
- Choline-Deficient L-Amino Acid-Defined (CDAA) Diet induced NASH Model
- High-Fat & High-Carbohydrate Diet induced NASH Model
- High-Fat & High-Cholesterol Diet induced NASH Model
- High-Fat & High-Cholesterol Diet & Fructose induced NASH Model
- High-Fat & Fructose induced NASH Model
- Diethylnitrosamine (DEN) & High-Fat & High-Carbohydrate Diet induced NASH Model
- High-Fat & CCL4 induced NASH Model
- Streptozotocin (STZ) & High-Fat induced NASH Model
- MC4R KO Mouse Model
- LDLR KO Mouse Model
Advantages
- Comprehensive Expertise: Our scientific team has in-depth expertise in liver disease models, offering tailored solutions for your research needs.
- Customizable Models: We provide customizable NASH models to suit your specific study, including adjustments in diet, dosing, and treatment protocols.
- Validated and Reliable Models: Our models are validated against the latest scientific literature, ensuring they closely mimic human NASH and provide reliable data for drug testing.
- Advanced Technologies: We utilize state-of-the-art technologies, such as immunohistochemistry, cytokine profiling, and gene expression analysis, to provide comprehensive data on drug efficacy.
- End-to-End Support: From experimental design to data interpretation, we offer complete support throughout your research project.
- Global Reach: Our services are available to academic institutions, pharmaceutical companies, and biotech firms worldwide.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What is the duration required to develop NASH in the High-Fat Diet induced NASH Model?
A: It typically takes 8-12 weeks of high-fat diet feeding to induce significant hepatic steatosis, inflammation, and fibrosis in the rodent model.
-
Q: Can the model be used to test drugs for cirrhosis and liver cancer?
A: Yes, the High-Fat Diet induced NASH Model is useful for testing drugs that may prevent the progression of NASH to cirrhosis and liver cancer.
-
Q: What are the limitations of this model?
A: While the model effectively simulates NASH, it may not fully replicate all aspects of human liver disease, particularly severe fibrosis and complications like portal hypertension.
-
Q: Do you offer custom services for model selection and experimental design?
A: Yes, our team can assist with selecting the appropriate model and designing experiments tailored to your specific research objectives.
Published Data
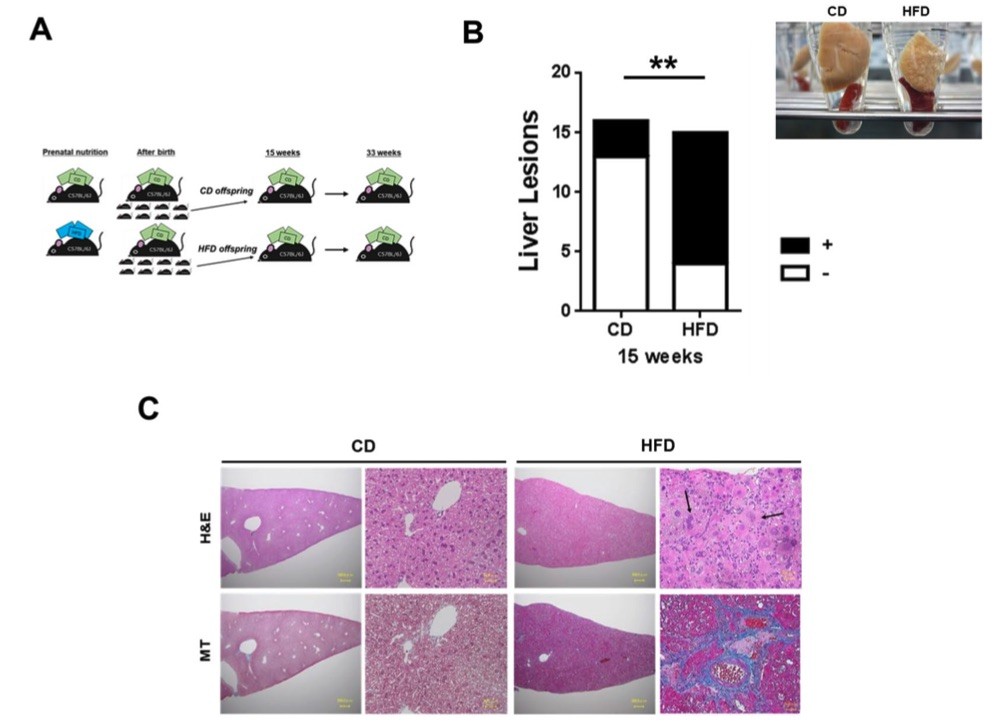 Fig. 2 Maternal HFD consumption induces hepatic steatosis in offspring.2
Fig. 2 Maternal HFD consumption induces hepatic steatosis in offspring.2
A novel mouse model was developed to investigate the impact of intrauterine overnutrition on liver pathophysiology in offspring. Female C57BL/6J mice, aged 8 weeks, were fed a high-fat diet (HFD) exclusively during the gestational period, distinguishing this study from previous models where maternal HFD exposure was sustained throughout the entire pregnancy and postpartum period (Fig. 2A). At 15 weeks of age, male offspring born to HFD-fed dams exhibited severe non-alcoholic steatohepatitis (NASH), along with splenomegaly (Fig. 2B). Maternal HFD intake significantly contributed to the development of liver lesions in the offspring at 15 weeks (p < 0.01, Fig. 2B). Histological analysis of liver sections from these offspring revealed signs of lobular inflammation (Fig. 2C), with atypical cells displaying marked dysplasia. These cells showed an increased nuclear-to-cytoplasmic ratio and enlarged nuclei, indicative of hyperploidization. Furthermore, fat accumulation, ductular reactions, and fibrosis were observed in the centrilobular zone (zone 3) of the liver, as evidenced by Masson's trichrome staining (blue staining, Fig. 2C).
References
- Eng, James M, and Jennifer L Estall. "Diet induced Models of Non-Alcoholic Fatty Liver Disease: Food for Thought on Sugar, Fat, and Cholesterol." Cells vol. 10,7 1805. 16 Jul. 2021, DOI:10.3390/cells10071805. Distributed under an Open Access license CC BY 4.0, without modification.
- Takiyama, Takao et al. "A maternal high-fat diet induces fetal origins of NASH-HCC in mice." Scientific Reports vol. 12,1 13136. 30 Jul. 2022, DOI:10.1038/s41598-022-17501-8. Distributed under an Open Access license CC BY 4.0, the image was modified by extracting and using only Parts A-C of the original image.
For Research Use Only.
