DOC-Na & Ammonia Water & Ethanol induced Chronic Atrophic Gastritis Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established animal models to evaluate the efficacy of drugs for Chronic Atrophic Gastritis. We provide customized solutions tailored to meet the specific requirements of your research, ensuring reliable and accurate results.
Introduction
Chronic atrophic gastritis (CAG) is a long-term inflammatory condition affecting the stomach lining, characterized by thinning and degeneration of the gastric mucosa. It is commonly caused by factors such as Helicobacter pylori infection, excessive alcohol consumption, or long-term use of non-steroidal anti-inflammatory drugs (NSAIDs). This progressive disease can lead to a reduction in gastric acid secretion, impaired digestion, and nutrient malabsorption. In the later stages, chronic inflammation in the stomach lining can result in gastric atrophy and increase the risk of gastric ulcers, bleeding, and even gastric cancer. CAG is typically associated with symptoms like bloating, indigestion, nausea, and abdominal discomfort, although it may be asymptomatic in some individuals. Early detection and intervention are essential to prevent further complications. Current treatment strategies include eradicating H. pylori infection, using proton pump inhibitors (PPIs) to reduce acid production, and making lifestyle changes such as avoiding alcohol and NSAIDs. Despite ongoing research, there are still no specific pharmacological treatments approved for CAG.
Disease Models and Applications
The DOC-Na & Ammonia Water & Ethanol-Induced Chronic Atrophic Gastritis Model is used to simulate the pathogenesis of chronic atrophic gastritis in rodents. In this model, chronic gastric injury is induced by administering deoxycholic acid sodium salt (DOC-Na), ammonia water, and ethanol to the animals. The combination of DOC-Na and ammonia causes damage to the gastric mucosa, while ethanol further exacerbates the injury by inducing inflammation and mucosal cell death. Over time, the model results in the characteristic features of atrophic gastritis, including thinning of the gastric mucosa, infiltration of inflammatory cells, and a reduction in gastric acid secretion. This model closely mimics human CAG, providing valuable insights into the mechanisms of disease progression. However, while this model is effective in replicating the injury and inflammation seen in CAG, it may not fully capture the long-term effects of H. pylori infection, which is a common cause of CAG in humans.
Simulates: The DOC-Na & Ammonia Water & Ethanol-Induced Chronic Atrophic Gastritis Model simulates the chronic inflammation and mucosal damage seen in human atrophic gastritis, helping researchers study the mechanisms and progression of the disease.
Evaluates Drugs: This model is used to evaluate the effectiveness of various therapeutic agents, including anti-inflammatory drugs, proton pump inhibitors (PPIs), and agents targeting gastric mucosal repair. It can also be used to assess potential treatments for reducing gastric mucosal injury and restoring normal gastric function.
Measurements
We offer a variety of measurements for evaluating drug efficacy in the DOC-Na & Ammonia Water & Ethanol-Induced Chronic Atrophic Gastritis Model, utilizing advanced technologies, including but not limited to:
- General observations: Body weight, food intake, mortality rate, and overall behavior.
- Gastric mucosal injury: Histopathological examination of gastric tissue for signs of mucosal erosion, thinning, and inflammatory cell infiltration.
- Cytokine profiling (e.g., ELISA): Measurement of inflammatory mediators such as TNF-α, IL-1β, and IL-6 to assess the extent of gastric inflammation.
- Gastric acid secretion: Evaluation of basal and stimulated gastric acid levels.
- Gene/protein expression profiling: RT-qPCR and Western blot analysis for key markers involved in inflammation and tissue repair, such as COX-2, IL-1β, and mucin.
- Gastric enzyme levels: Measurement of serum pepsinogen and gastric mucosal integrity markers.
In addition to the established disease models, our scientific team can develop custom models tailored to your research needs. We provide expert assistance in experimental design, model selection, and data analysis to ensure a comprehensive approach to your project.
Advantages
- Expertise: Our team has extensive experience in developing and optimizing animal models for digestive diseases like chronic atrophic gastritis.
- Customization: We offer flexible and customized model development to suit your specific research objectives.
- Comprehensive services: From model creation to data analysis, we provide end-to-end support to ensure high-quality, reliable results.
- State-of-the-art technology: We employ advanced techniques such as gene/protein expression analysis, histopathology, and cytokine profiling to ensure accurate and detailed measurements.
- Scientific collaboration: Our team works closely with clients to assist with experimental design, model selection, and data interpretation, ensuring your project's success.
- Reliable results: Our rigorous quality control ensures reproducible and reliable data, giving you confidence in your research findings.
Case Study
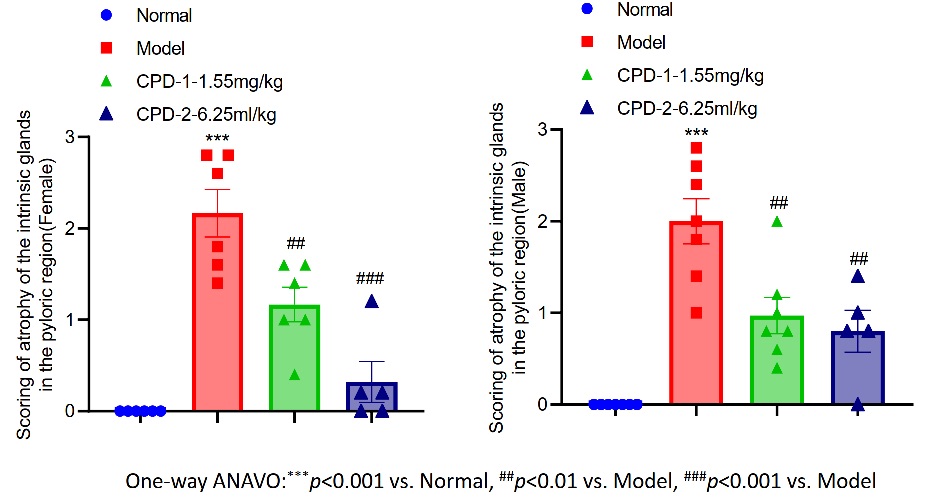 Fig.1 Scoring of atrophy of the intrinsic glands in the pyloric region.
Fig.1 Scoring of atrophy of the intrinsic glands in the pyloric region.
This study assessed the therapeutic efficacy of CPD on atrophy of the intrinsic glands in the pyloric region using a model animal system. The findings revealed that in both female and male animals, the model group displayed significantly elevated atrophy scores of the intrinsic glands in the pyloric region compared to the normal control group, confirming the successful induction of glandular atrophy. Conversely, both CPD-treated groups exhibited a notable reduction in atrophy scores when compared to the model group. Notably, the CPD-1 (1.55 mg/kg) group showed a marked decrease in scores, whereas the CPD-2 (6.25 ml/kg) group demonstrated an even more substantial improvement. These results indicate that CPD effectively mitigates atrophy of the intrinsic glands in the pyloric region in a dose-dependent manner, underscoring its potential as a therapeutic agent for related diseases.
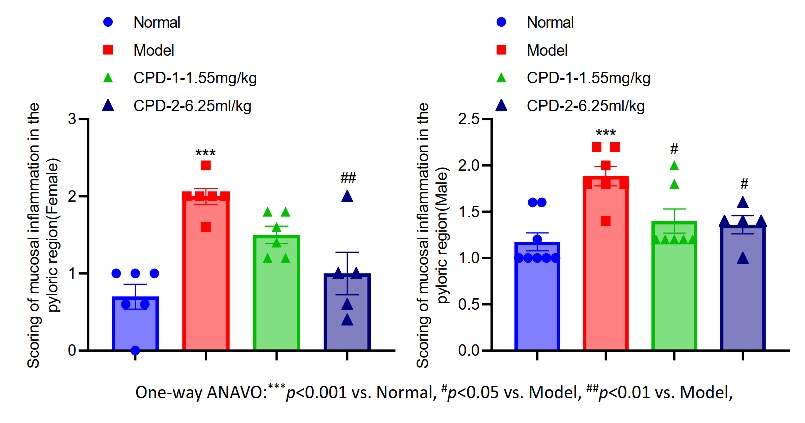 Fig.2 Scoring of mucosal inflammation in the pyloric region.
Fig.2 Scoring of mucosal inflammation in the pyloric region.
This study aimed to evaluate the ameliorative effects of CPD on mucosal inflammation in the pyloric region in a model animal system. The results showed that, compared with the normal control group, the mucosal inflammation scores in the pyloric region were significantly elevated in both female and male animals of the model group, indicating the successful induction of significant mucosal inflammation. Following CPD intervention, the inflammation scores were significantly reduced in both sexes, with the high-dose group (6.25 ml/kg) exhibiting a more pronounced anti-inflammatory effect. The scores decreased to approximately 1.0 in both female and male animals, significantly lower than those in the model group. These findings suggest that CPD can effectively alleviate mucosal inflammation in the pyloric region in a dose-dependent manner, highlighting its potential therapeutic value for related inflammatory diseases.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What is Chronic Atrophic Gastritis (CAG)?
A1: CAG is a condition characterized by long-term inflammation and thinning of the stomach lining, which can lead to gastric ulcers or cancer.
-
Q2: How is the DOC-Na & Ammonia Water & Ethanol-Induced Chronic Atrophic Gastritis Model created?
A2: The model is induced by administering DOC-Na, ammonia water, and ethanol to rodents, causing gastric injury, inflammation, and mucosal thinning.
-
Q3: What drugs can be evaluated using this model?
A3: Drugs such as proton pump inhibitors (PPIs), anti-inflammatory agents, and mucosal repair therapies can be tested for their efficacy in this model.
-
Q4: What measurements are used to evaluate drug efficacy?
A4: Key measurements include histopathological examination, cytokine profiling, gastric acid secretion levels, and gene expression analysis related to inflammation and mucosal repair.
-
Q5: Can you develop customized models for specific research needs?
A5: Yes, we offer customized models and expert guidance to suit your specific research objectives and help you achieve reliable results.
For Research Use Only.
