Folic Acid induced Acute Kidney Injury Modeling & Pharmacodynamics Service
Creative Biolabs offers a wide range of well-established models to evaluate the efficacy of treatments for acute kidney injury. These models provide valuable insights into drug effectiveness, enabling the development of effective therapeutic strategies.
Introduction
Acute Kidney Injury (AKI), formerly known as acute renal failure, is a rapid decline in kidney function, typically occurring within hours or days. It is characterized by an increase in serum creatinine, a decrease in urine output, and electrolyte imbalances. AKI can be caused by a variety of factors, including ischemia, nephrotoxins, sepsis, and major surgeries. It is a serious condition with high morbidity and mortality rates, especially when left untreated. AKI can progress to chronic kidney disease (CKD) if not managed effectively, leading to long-term renal dysfunction. The pathophysiology of AKI involves a complex interaction between renal tubules, glomeruli, and the vascular system. Early diagnosis and intervention are crucial to prevent further kidney damage and to improve patient outcomes.
Folic Acid-Induced Acute Kidney Injury Model
The Folic Acid-Induced Acute Kidney Injury Model is established by administering folic acid to rodents, which induces renal tubular toxicity and subsequent AKI. This model is particularly useful for studying the mechanisms of renal damage, inflammation, and fibrosis. The model shows histological features like tubular necrosis and infiltration of inflammatory cells, making it valuable for testing nephroprotective drugs. It is advantageous in evaluating potential therapies that target inflammation, oxidative stress, and cell apoptosis. However, one limitation is its potential variability in response depending on dosage and rodent strain.
- Simulates: It is commonly used to simulate drug-induced AKI and evaluate mechanisms of kidney damage.
- Evaluates Drugs: It is used to evaluate nephroprotective drugs, anti-inflammatory agents, and agents targeting oxidative stress.
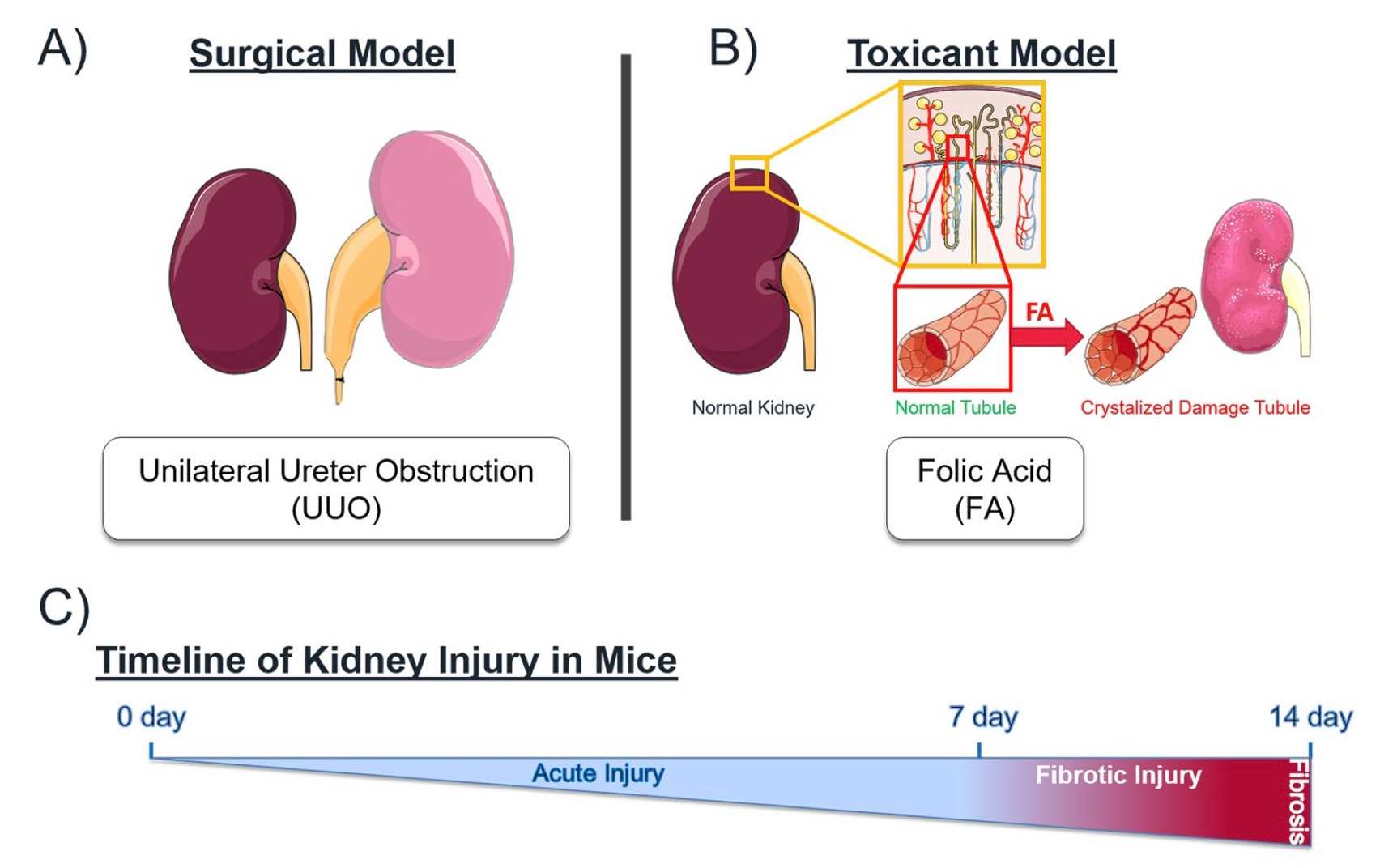 Fig. 1 Mechanistically distinct mouse models of kidney injury.1,3
Fig. 1 Mechanistically distinct mouse models of kidney injury.1,3
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in the Folic Acid-Induced Acute Kidney Injury model, utilizing advanced technologies, including but not limited to:- General observations: Body weight, urine output, mortality rate, renal function (e.g., serum creatinine, blood urea nitrogen).
- Histology: Kidney tissue analysis, including necrosis, apoptosis, and fibrosis.
- Immunohistochemistry: Detection of inflammatory markers and immune cell infiltration in renal tissues.
- Cytokine profiling (e.g., ELISA): Quantification of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6.
- Serum biomarkers: Renal function markers like creatinine, BUN, and electrolytes.
- Gene/protein expression profiling: RT-qPCR and Western blot to assess gene expression related to kidney injury, fibrosis, and inflammation.
Our scientific team can assist with experimental design and data analysis, ensuring a customized approach to your project.
Related Services
In addition to the Folic Acid-Induced Acute Kidney Injury Model, we also offer services for other models of acute kidney injury. These models complement our folic acid model and provide a broad range of options to explore kidney damage and therapeutic interventions.
- Gentamicin-Induced Acute Renal Failure Model
- Cisplatin-Induced Acute Renal Injury Model
- Glycerol-Induced Acute Renal Failure Model
- Contrast Agent-Induced Acute Kidney Injury Model
- Lipopolysaccharide (LPS)-Induced Acute Kidney Injury Model
- Cecal Ligation and Puncture (CLP)-Induced Acute Kidney Injury Model
Our advantages
- Comprehensive Expertise: We specialize in developing and optimizing animal models tailored to your research needs, ensuring the highest level of precision in AKI studies.
- Customized Solutions: Our team works closely with clients to adapt models for specific drug evaluation, allowing for personalized experimental designs.
- Cutting-Edge Technology: We employ advanced techniques such as immunohistochemistry, cytokine profiling, and gene expression analysis, ensuring accurate and reliable results.
- Efficient Turnaround: With our streamlined processes, we offer quick model setup and timely data delivery, helping you meet research deadlines.
- Full-Spectrum Support: From initial model development to final data interpretation, our experienced team provides continuous support throughout the study.
- Robust Data Quality: We prioritize data integrity, ensuring high reproducibility and reliability in all experimental results, facilitating meaningful conclusions for your research.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What are the common applications of the Folic Acid-Induced AKI Model?
This model is widely used to study nephrotoxicity, evaluate kidney injury mechanisms, and test nephroprotective drugs.
-
2. How long does it take to establish the Folic Acid-Induced AKI Model?
The model can be established within a few days, with the acute phase of kidney injury observed within 24-48 hours after administration.
-
3. Can this model simulate chronic kidney injury?
While primarily used for acute kidney injury, modifications to the protocol can simulate chronic kidney damage by allowing prolonged observation.
-
4. What are the key measurements for evaluating drug efficacy in this model?
Key measurements include serum creatinine, BUN, cytokine levels, histological changes, and gene expression profiling.
Published Data
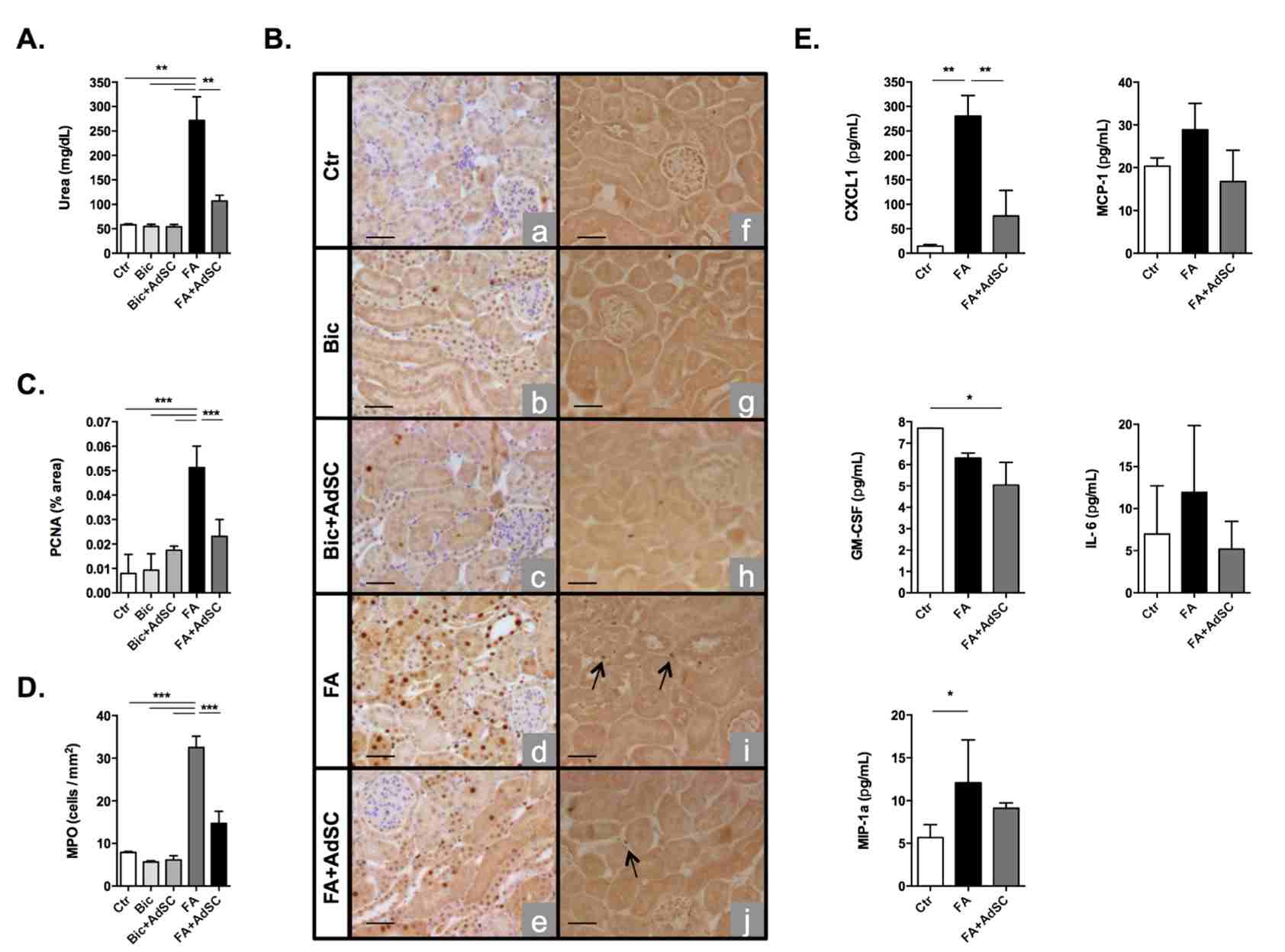 Fig 2. Folic Acid-induced acute kidney injury.2,3
Fig 2. Folic Acid-induced acute kidney injury.2,3
The effect of AdSC treatment on a mouse model of folic acid (FA)-induced acute kidney injury (AKI) was evaluated by administering 200 mg/kg of FA to FVB mice via intraperitoneal injection, followed by syngeneic AdSC therapy 24 hours later. FA treatment significantly impaired kidney function, as shown by increased serum urea levels, whereas AdSC therapy protected the kidneys, reducing serum urea levels 48 hours post-treatment (Fig. 2A). FA primarily targets kidney tubule cells, inducing abnormal cell proliferation, as indicated by elevated proliferating cell nuclear antigen (PCNA) expression. AdSC treatment restored PCNA expression to baseline levels, preserving the normal state of tubule cells (Fig. 2B and 2C). Inflammation and immune cell infiltration are key features of AKI, and MPO expression was significantly reduced in the AdSC-treated group compared to untreated mice (Fig. 2B and 2D). Bioplex proteomic analysis revealed increased levels of inflammatory cytokines, including Chemokine (C-X-C motif) ligand 1 (CXCL-1) and macrophage inflammatory protein 1 alpha (MIP-1α) after FA injury, with AdSC treatment significantly reducing CXCL-1 and Granulocyte-macrophage colony-stimulating factor (GM-CSF) levels, indicating a reduction in the inflammatory response (Fig. 2E).
References
- Feng, Daniel et al. "Characterization of Matricellular Protein Expression Signatures in Mechanistically Diverse Mouse Models of Kidney Injury." Scientific Reports vol. 9,1 16736. 13 Nov. 2019. https://doi.org/10.1371/journal.pone.0142183
- Burgos-Silva, Marina et al. "Adipose Tissue-Derived Stem Cells Reduce Acute and Chronic Kidney Damage in Mice." PloS One vol. 10,11 e0142183. 13 Nov. 2015. https://doi.org/10.1371/journal.pone.0142183
- Distributed under an Open Access license CC BY 4.0, without modification
For Research Use Only.
