Arteriovenous Fistula Thrombosis Modeling & Pharmacodynamics Service
Introduction
Thrombosis, the formation of blood clots within blood vessels, is a critical pathological process underlying numerous cardiovascular diseases, including the devastating complications seen in patients with end-stage renal disease (ESRD). For individuals relying on hemodialysis, arteriovenous fistula (AVF) thrombosis represents a major cause of access failure, leading to significant morbidity and increased healthcare burdens. Understanding the complex mechanisms driving these thrombotic events is paramount for developing effective interventions.
Creative Biolabs is equipped to provide a variety of well-established rodent thrombosis models to rigorously evaluate the efficacy of novel anti-thrombotic and pro-patency therapies.
Arteriovenous Fistula Thrombosis Model
The arteriovenous fistula (AVF) thrombosis model is a highly translational preclinical tool designed to mimic the pathophysiology of AVF failure and thrombosis in a controlled laboratory setting. This model is indispensable for investigating the intricate biological processes involved in AVF maturation, intimal hyperplasia, and subsequent thrombotic occlusion. It serves as a robust platform for identifying novel therapeutic targets, evaluating the efficacy of pharmacological agents aimed at improving AVF patency, and testing innovative surgical techniques or device interventions.
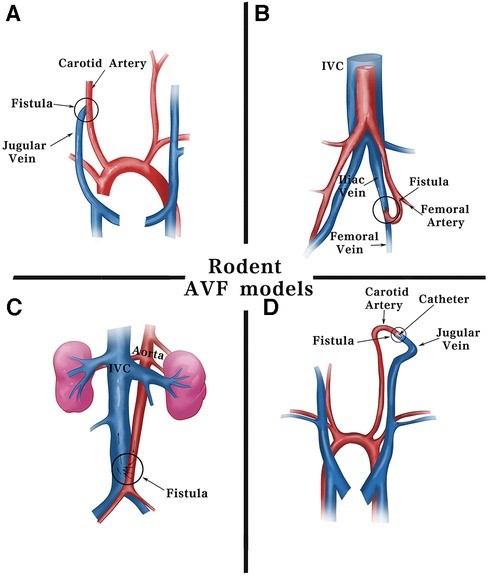 Fig.1 Surgical methods for fistula models in rodents.1,3
Fig.1 Surgical methods for fistula models in rodents.1,3
Model Construction Steps
The construction of our AVF thrombosis model primarily involves precise microsurgical techniques to create an anastomosis between an artery and a vein in small animal species, typically rodents. This strategy ensures a consistent and reproducible model for studying AVF patency and thrombosis.
01Anesthesia and Preparation
The animal is anesthetized, and the surgical site (e.g., neck for carotid-jugular fistula, or groin for femoral AVF) is meticulously prepared and sterilized.
02Vessel Isolation
Under a surgical microscope, the target artery (e.g., common carotid artery) and vein (e.g., jugular vein) are carefully isolated from surrounding tissues.
03Anastomosis Creation
A precise end-to-side or side-to-side anastomosis is created using fine sutures. Our primary method is surgical suturing, though we are proficient in other techniques like needle puncture or cuff methods, depending on specific research requirements.
04Flow Establishment
Upon completion of the anastomosis, blood flow through the newly created fistula is carefully established and confirmed.
05Closure and Recovery
The surgical site is closed, and the animal is monitored closely during recovery, with appropriate analgesia administered.
06Thrombosis Induction (Optional)
For induced thrombosis studies, specific methods such as partial ligation of the outflow vein, local vessel injury, or systemic pro-thrombotic challenges may be applied at a defined time point post-fistula creation.
Strengths and Limitations
Strengths:
- High Translational Relevance: Faithfully recapitulates key pathophysiological events observed in human AVF failure, including intimal hyperplasia, inflammation, and thrombosis.
- Controlled Environment: Allows for systematic investigation of specific mechanisms and the evaluation of therapeutic interventions in a controlled setting.
- Comprehensive Endpoint Analysis: Enables a wide range of assessments, from patency and hemodynamics to detailed histological and molecular analyses.
- Customizable: Adaptable to study spontaneous or induced thrombosis, and various therapeutic approaches.
Limitations:
- Species Differences: Rodent physiology, while similar, is not identical to human, which can lead to some translational discrepancies.
- Surgical Skill Dependency: Requires highly skilled microsurgeons to ensure consistent and reproducible fistula creation.
- Ethical Considerations: Requires strict adherence to animal welfare guidelines and ethical protocols.
Evaluation Platform
Creative Biolabs offers a comprehensive evaluation platform to assess the efficacy of interventions in the AVF thrombosis model, utilizing advanced instruments across biochemical, molecular, cellular, histopathological, and imaging disciplines. Key test indicators include:
- Fistula Patency (flow, diameter)
- Neointimal Hyperplasia
- Thrombus Burden
- Inflammation Markers
- Endothelial Function
- Smooth Muscle Cell Proliferation
- Gene/Protein Expression Profiles (vascular remodeling, coagulation)
Applications
- Simulating Diseases: It effectively simulates critical complications of ESRD related to vascular access, including AVF maturation failure, stenosis, thrombotic occlusion, and broader vascular remodeling disorders.
- Evaluating Drugs: This model is ideal for assessing various pharmacological agents, such as anti-thrombotic (anticoagulants, antiplatelets), anti-proliferative, anti-inflammatory compounds, and drugs specifically targeting endothelial dysfunction or promoting vascular maturation.
- Testing Treatments: It serves as a robust platform for evaluating diverse therapeutic approaches, including pharmacological interventions, novel surgical techniques to enhance AVF patency, gene therapies for vascular remodeling, cell-based therapies for vascular repair, and innovative device-based interventions for improved vascular access.
Related Thrombosis Models
- Transient Blood Flow Occlusion induced Inferior Vena Cava Thrombosis Model
- Thrombin induced Inferior Vena Cava Thrombosis Model
- Fe2O3 induced Arterial Thrombosis Model
- Foreign Matter induced Arterial Thrombosis Model
- Ferric Chloride induced Thrombosis Model
Our Advantages
- Expertise: Years of experience in preclinical vascular biology and thrombosis research.
- Scientific Rigor: Meticulous experimental design, execution, and data analysis for reliable, high-quality results.
- Comprehensive Services: Full-spectrum capabilities from model development to advanced endpoint analyses.
- Translational Focus: Generating data highly predictive of clinical outcomes to accelerate your drug development.
- Ethical Commitment: Strict adherence to the "3R" principle and IACUC oversight for humane and compliant studies.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs provides unparalleled expertise and comprehensive services of the AVF thrombosis model to support your research endeavors. Partner with us to accelerate your therapeutic development and improve patient outcomes. Contact us today to discuss your project requirements.
FAQs
-
Q1: How do you ensure the reproducibility of its AVF thrombosis model?
A: Reproducibility is paramount in our preclinical studies. We achieve this through standardized surgical protocols, highly experienced microsurgeons, rigorous animal selection criteria, and consistent post-operative care. Furthermore, our comprehensive quality control measures, including regular calibration of equipment and blinded data analysis, contribute significantly to the reliability and consistency of our model outcomes.
-
Q2: Can your AVF thrombosis model be customized to evaluate specific drug delivery methods?
A: Absolutely. Our AVF thrombosis model is highly adaptable to accommodate various drug delivery methods. We can explore systemic administration (e.g., oral, intravenous, subcutaneous), local delivery techniques (e.g., perivascular application, direct injection into the fistula), or even integrate specialized drug-eluting devices. We collaborate with clients to tailor the study design to their unique compound and delivery strategy.
-
Q3: What are the key indicators you assess to determine AVF patency and thrombosis?
A: We employ a multi-modal approach to assess AVF patency and thrombosis. Primary indicators include non-invasive Doppler ultrasound for blood flow velocity and vessel diameter, visual inspection, and palpation for thrill and bruit. For more detailed analysis, we perform micro-angiography, histopathological examination to quantify neointimal hyperplasia and thrombus burden, and molecular analyses of relevant biomarkers.
-
Q4: Can your model differentiate between mechanisms of AVF maturation failure and late-stage thrombosis?
A: Yes, our model is designed to investigate both early-stage AVF maturation failure and later-stage thrombotic events. By varying the study duration and employing specific induction methods, we can dissect the distinct molecular and cellular processes contributing to initial non-maturation (often driven by intimal hyperplasia and remodeling) versus acute thrombotic occlusion. This allows for targeted therapeutic development.
-
Q5: What kind of pre-study consultation do you offer for the AVF thrombosis model?
A: We offer extensive pre-study consultation to ensure your project is optimally designed. This includes in-depth discussions on your research goals, compound characteristics, potential mechanisms of action, and desired endpoints. Our scientific team provides expert guidance on model selection, experimental design, sample size calculations, and analytical strategies to maximize the success of your study.
-
Q6: Beyond standard assessments, can you perform specialized analyses like flow cytometry on AVF tissue?
A: Absolutely. In addition to our standard comprehensive assessments, Creative Biolabs is equipped to perform specialized analyses such as flow cytometry on isolated AVF tissue or circulating blood. This allows for detailed characterization of immune cell populations, activation states, and other cellular parameters, providing deeper mechanistic insights into the inflammatory and cellular components of AVF pathology.
Published Data
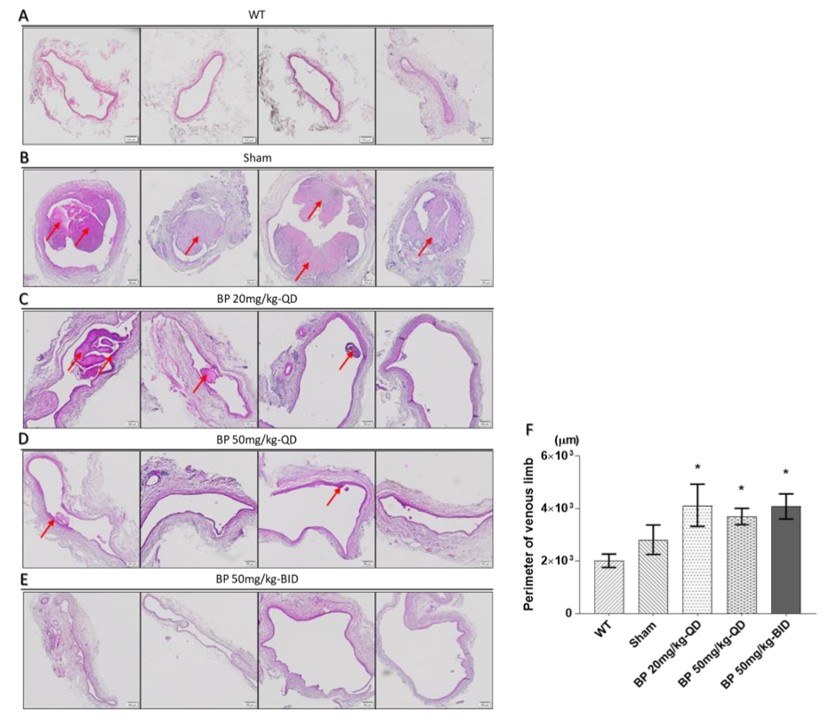 Fig.2 Effect of BP on thrombosis and neointimal hyperplasia of AVF rat model.2,3
Fig.2 Effect of BP on thrombosis and neointimal hyperplasia of AVF rat model.2,3
This demonstrated that N-butylidenephthalide (BP) effectively prevented AVF stenosis in a rat model by inhibiting the phenotypic switch of vascular smooth muscle cells (VSMCs) via AMPK activation. The project results showcased the model's effectiveness in evaluating novel therapeutic compounds and provided valuable insights into the molecular mechanisms underlying AVF stenosis.
References
- Li, Yuxuan et al. "The rodent models of arteriovenous fistula." Frontiers in cardiovascular medicine vol. 11 1293568. 18 Jan. 2024. https://doi.org/10.3389/fcvm.2024.1293568
- Yang, Hsin-Han et al. "N-Butylidenephthalide Inhibits the Phenotypic Switch of VSMCs through Activation of AMPK and Prevents Stenosis in an Arteriovenous Fistula Rat Model." International journal of molecular sciences vol. 21,19 7403. 7 Oct. 2020. https://doi.org/10.3390/ijms21197403
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
