Ethanol induced Gastric Ulcer Modeling & Pharmacodynamics Service
Introduction
Gastric ulcers are a type of peptic ulcer, which are sores that form in the lining of the stomach or the upper part of the small intestine. They are commonly caused by an imbalance between aggressive factors, such as stomach acid, and protective factors, like the mucus lining. The most frequent causes of gastric ulcers include infection with Helicobacter pylori bacteria, excessive use of nonsteroidal anti-inflammatory drugs (NSAIDs), and stress. Symptoms include burning stomach pain, bloating, nausea, and in some cases, bleeding. If untreated, gastric ulcers can lead to complications like perforation, obstruction, or bleeding, making early diagnosis and treatment crucial. To evaluate the efficacy of potential gastric ulcer treatments, Creative Biolabs offers a variety of well-established rodent models. These models mimic the pathophysiological features of gastric ulcers, providing a reliable platform for drug testing and development. Our services include comprehensive preclinical studies to assess the effectiveness of therapeutic candidates under controlled conditions.
Disease Models and Applications
The ethanol-induced rodent gastric ulcer model is commonly used to study the pathogenesis of gastric ulcers and to evaluate therapeutic interventions. This model is created by orally administering ethanol to rodents, which induces oxidative stress, inflammation, and damage to the gastric mucosa, leading to ulcer formation. Ethanol causes a rapid reduction in the protective mucus layer and increases gastric acid secretion, mimicking the human condition of alcohol-induced gastric ulcers. The model is characterized by its reproducibility, ease of administration, and similarity to human ulceration mechanisms, making it highly valuable for drug testing. Its primary advantage is that it offers a clear, acute gastric injury, which allows for the assessment of rapid therapeutic effects. However, a limitation of this model is that it primarily focuses on alcohol-induced ulceration and may not fully represent ulcers caused by H. pylori infection or NSAIDs. Additionally, the severity of the ulcers may vary depending on the ethanol dose and the rodent strain used, which could affect the consistency of results. Despite these drawbacks, the Ethanol-Induced Rodent Gastric Ulcer Model remains an essential tool in preclinical ulcer research.
- Simulates: This model replicates the pathophysiological conditions associated with alcohol-induced gastric mucosal damage, providing insight into ulcer formation mechanisms.
- Evaluates Drugs: This model is used to evaluate drugs aimed at treating alcohol-induced gastric ulcers, including anti-ulcer agents such as proton pump inhibitors (PPIs), H2-receptor antagonists, mucosal protectants, and antioxidant therapies.
Measurements
We offer a comprehensive range of measurements for evaluating drug efficacy in the ethanol-induced rodent gastric ulcer model, utilizing advanced techniques, including but not limited to:
- General observations: Body weight, ulcer area measurement, mortality rate, food/water intake, and signs of gastric distress.
- Endoscopic evaluation: Visualization and measurement of ulcer size and severity in the gastric mucosa.
- Histopathological analysis: Assessment of gastric tissue damage, including mucosal erosion, inflammation, and hemorrhage through hematoxylin and eosin (H&E) staining.
- Cytokine profiling (e.g., ELISA): Expression levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which are elevated in ulceration.
- Oxidative stress markers: Measurement of reactive oxygen species (ROS) and antioxidant enzymes like superoxide dismutase (SOD), catalase, and glutathione peroxidase.
- Gene/protein expression profiling via RT qPCR and Western blot techniques: Expression of gastric injury markers, such as COX-2, MMPs, and mucin proteins, to assess the degree of tissue repair and inflammation.
In addition to the established model, our expertise also includes the development of customized rodent models based on literature and prior studies. Our scientific team is available for guidance in experimental design, model selection, and data analysis, ensuring a tailored and effective approach for your specific research needs.
Related Services
In addition to the ethanol-induced rodent gastric ulcer model, we also provide other methods to induce gastric ulcers, offering a broader range of research options.
Advantages
- Expertise and Experience: With years of specialized knowledge in preclinical models, we offer high-quality, reliable, and scientifically sound models for gastric ulcer research.
- Comprehensive Services: From experimental design and model selection to data analysis, we provide full support throughout your project, ensuring a tailored approach.
- Cutting-Edge Technologies: Our advanced technologies, including cytokine profiling, histopathological analysis, and gene/protein expression profiling, ensure accurate and meaningful results.
- Customized Models: In addition to established models, we have the flexibility to develop novel, customized models based on your specific research needs.
- Collaborative Approach: Our scientific team works closely with you, ensuring that each phase of the study aligns with your research goals.
- Reliable Results: Our rigorous quality control and standardized procedures ensure reproducible and consistent results, helping you achieve reliable data for drug evaluation.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: How long does it take to conduct a study using your gastric ulcer models?
A1: The duration of the study depends on the specific model and the scope of your project. Typically, a preclinical study can range from a few weeks to a couple of months, including data collection and analysis.
-
Q2: What support do you provide during the study?
A2: Our scientific team provides full support, including experimental design, model selection, data analysis, and interpretation, ensuring a seamless process from start to finish.
-
Q3: Do you offer other disease models beyond gastric ulcers?
A3: Yes, we offer a variety of rodent disease models, including those for liver disease, cancer, atherosclerosis, and kidney fibrosis, among others.
-
Q4: How do I get started with your services?
A4: Simply contact us to discuss your research goals. Our team will guide you through the model selection, study design, and timeline to get your project underway.
-
Q5: Are your models validated for publication?
A5: Yes, all our models are validated based on well-established scientific protocols and are suitable for generating publishable results.
Published Data
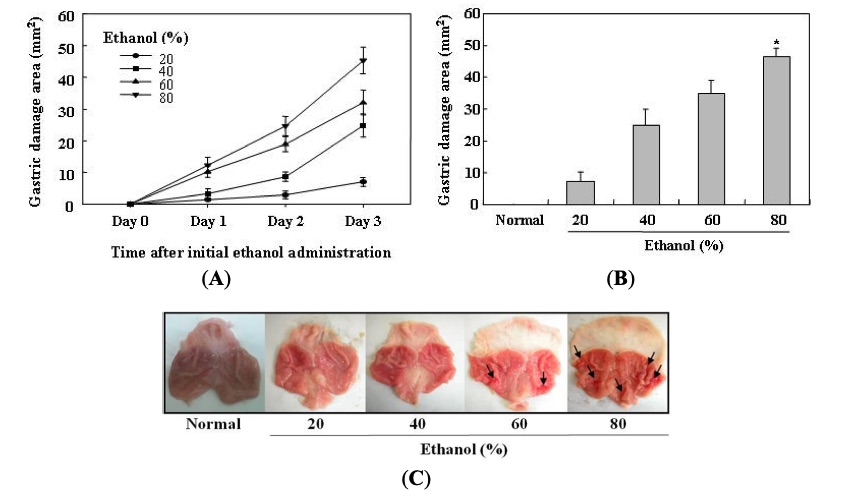 Fig. 1 Determination of optimal ethanol-induced gastric mucosal injury model in rats.1
Fig. 1 Determination of optimal ethanol-induced gastric mucosal injury model in rats.1
In this study, gastric mucosal lesions in rats were induced using ethanol to assess the gastroprotective effects of selenium. To determine the optimal dose of ethanol for inducing gastric mucosal damage, rats were administered varying concentrations of ethanol (20%, 40%, 60%, and 80%) over a period of 3 days. Gastric lesions were evaluated macroscopically based on the depth of penetration into the gastric mucosal surface. As demonstrated in Figure 1, ethanol administration for 3 days resulted in a dose-dependent increase in the area of gastric damage across all treatment groups. In contrast, ethanol treatment for 1 or 2 days resulted in relatively smaller gastric damage areas. Notably, the highest degree of gastric damage was observed with 80% ethanol, which produced the largest area of damage after 3 days of administration. Gastric mucosal lesions such as erosions, bleeding, and ulcers were clearly visible in rats treated with 80% ethanol for 3 days. Based on these observations, 80% ethanol administered for 3 days was established as the optimal condition for studying the protective effects of selenium.
Reference
- Kim, Jeong-Hwan et al. "Gastroprotective effect of selenium on ethanol-induced gastric damage in rats." International Journal of Molecular Sciences vol. 13,5 (2012): 5740-5750. doi:10.3390/ijms13055740. Distributed under an Open Access license CC BY 3.0, without modification.
For Research Use Only.
