5/6 Nephrectomy induced Hypertension Modeling & Pharmacodynamics Service
At Creative Biolabs, we are committed to advancing hypertension research by providing a diverse array of well-established and meticulously characterized rodent models, enabling comprehensive evaluation of novel therapeutic agents.
Introduction
Hypertension, a pervasive and often asymptomatic condition, stands as a primary driver of global cardiovascular morbidity and mortality. Its intricate pathophysiology, involving renal, vascular, and neurohumoral mechanisms, necessitates sophisticated preclinical models for effective drug discovery.
5/6 Nephrectomy-Induced Hypertension Model
The 5/6 Nephrectomy (Nx) model, also recognized as the subtotal Nx model, represents a highly validated and indispensable experimental paradigm for inducing chronic hypertension and progressive renal dysfunction. This model faithfully replicates the complex pathophysiological cascade observed in patients with diminished renal mass, offering a robust platform to investigate the interplay between kidney injury and systemic blood pressure regulation, as well as the progression of chronic kidney disease (CKD).
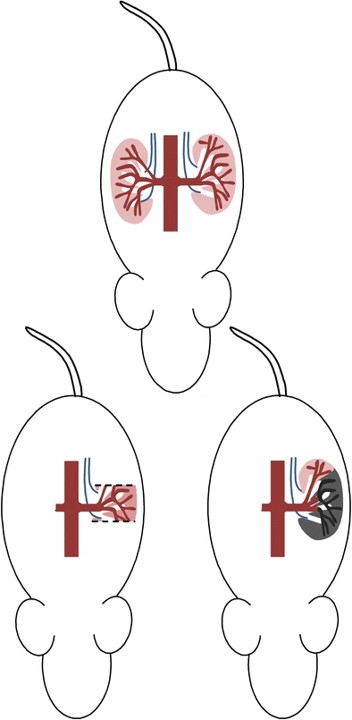 Fig.1 Schematic diagram of 5/6 Nx operation.1,3
Fig.1 Schematic diagram of 5/6 Nx operation.1,3
Model Construction Steps
The construction of the 5/6 Nx model involves surgically reducing approximately 5/6ths of the total functional renal mass. This is typically achieved through a two-stage surgical procedure, allowing for animal recovery between interventions.
01Stage 1 (Partial Nx)
One kidney is exposed, and either two-thirds of its mass is rendered non-functional via ligation and infarction of its arterial supply, or two-thirds is surgically resected.
02Recovery Period
Animals are allowed a period of recovery (typically one week) to stabilize.
03Stage 2 (Contralateral Nx)
The contralateral (remaining) kidney is surgically removed, completing the 5/6 reduction in renal mass.
Strengths and Limitations
Strengths:
- High Clinical Relevance: Closely mimics human renal parenchymal disease-induced hypertension and CKD progression.
- Chronic Hypertension: Induces sustained hypertension, suitable for long-term efficacy and safety studies.
- Multi-Organ Impact: Allows investigation of hypertension's effects on the heart, brain, and vasculature.
- Reproducibility: Standardized surgical techniques ensure consistent and reliable outcomes.
- Therapeutic Window: Progressive disease offers clear opportunities for intervention at various stages.
- Species Versatility: Successfully applicable in both rat and mouse models, allowing for genetic manipulation studies in mice.
Limitations:
- Surgical Complexity: Requires skilled surgical expertise to ensure consistent renal mass reduction and minimize mortality.
- Post-Operative Care: Demands meticulous post-operative care to manage potential complications.
- Variability (Method-Dependent): The onset and severity of hypertension can vary depending on the specific surgical method (infarction vs. polectomy).
Evaluation Platform
Creative Biolabs offers a state-of-the-art evaluation platform to comprehensively assess the efficacy of novel compounds in the 5/6 Nx model. We provide comprehensive analyses spanning biochemical, molecular, cellular, histopathological, behavioral, and advanced imaging.
Key Test Parameters:
- Continuous telemetry-based blood pressure and heart rate monitoring.
- Renal function markers: creatinine, blood urea nitrogen (BUN), proteinuria, and glomerular filtration rate (GFR).
- Histopathological scoring: assessment of glomerulosclerosis, fibrosis, and inflammation.
- Cardiac hypertrophy and vascular remodeling evaluations.
- Quantification of relevant biomarkers.
Applications
- Disease Modeling and Pathophysiological Understanding: This model accurately simulates the progression of CKD, renal parenchymal hypertension, and associated cardiovascular complications like cardiac hypertrophy and vascular dysfunction, providing a robust platform to unravel the underlying mechanisms of these conditions.
- Therapeutic Efficacy and Safety Evaluation: It serves as a critical tool for assessing the effectiveness and safety of a wide range of pharmacological agents, including antihypertensives, renoprotective compounds, and drugs targeting metabolic disturbances or cardiovascular remodeling.
- Investigating Diverse Treatment Modalities: Beyond traditional pharmacology, the model facilitates the exploration of novel treatment approaches, such as dietary modifications, cellular therapies, and gene therapies, offering insights into their potential to mitigate disease progression and improve outcomes.
Related Hypertension Models
Our Advantages
- Decades of Expertise: Unparalleled experience in rodent models of cardiovascular and renal diseases.
- Precision and Consistency: Meticulous surgical techniques ensure high reproducibility and minimal variability.
- Comprehensive Phenotyping: Extensive in-house capabilities for multi-dimensional data collection.
- Customized Study Design: Flexible and tailored experimental protocols to meet unique research goals.
- Integrated Data Analysis: Expert interpretation and robust reporting for actionable insights.
- Ethical Animal Care: Adherence to the highest standards of animal welfare and humane practices.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is your trusted partner in navigating the complexities of hypertension research. Leveraging our profound expertise and advanced capabilities in the 5/6 Nx model, we are poised to support your journey from target validation to preclinical efficacy. Reach out to us today to explore how our specialized services can accelerate your drug discovery initiatives.
FAQs
-
Q1: Can you customize the 5/6 Nx model for specific research objectives or genetic backgrounds?
A: Absolutely. Our team excels in designing tailored studies. We can adapt the model to various rodent strains, including those with specific genetic modifications, and incorporate additional challenges like high-salt diets or co-morbidities to align precisely with your unique research questions and desired disease phenotypes.
-
Q2: How is blood pressure monitored in the 5/6 Nx model at Creative Biolabs?
A: We primarily utilize state-of-the-art telemetry systems for continuous, conscious blood pressure monitoring. This advanced method provides highly accurate and stress-free measurements, capturing real-time fluctuations and ensuring reliable assessment of antihypertensive drug efficacy without the confounding effects of restraint or anesthesia.
-
Q3: What are the common challenges associated with the 5/6 Nx model, and how does Creative Biolabs address them?
A: Key challenges include surgical mortality, variability in renal mass reduction, and managing post-operative complications. Creative Biolabs mitigates these through highly experienced surgical teams, standardized protocols, rigorous animal health monitoring, and meticulous post-operative care, ensuring high model success rates and data consistency.
-
Q4: What specific endpoints and parameters are typically included in a standard 5/6 Nx study?
A: A standard study generally includes comprehensive physiological and pathological assessments. These encompass continuous blood pressure via telemetry, renal function markers (serum creatinine, BUN, proteinuria, GFR), kidney and heart histopathology (fibrosis, inflammation, glomerulosclerosis), organ weights, and relevant circulating biomarkers.
-
Q5: Can clients provide their own test compounds for evaluation in the 5/6 Nx model?
A: We welcome client-supplied compounds for evaluation. Our team works closely with you to determine appropriate dosing regimens, routes of administration (e.g., oral, subcutaneous, intravenous), and treatment durations, ensuring optimal experimental design for assessing your compound's efficacy and safety.
-
Q6: How do you ensure the reproducibility and consistency of the 5/6 Nx model?
A: Reproducibility is paramount. We achieve this through highly standardized surgical procedures performed by expert technicians, consistent animal sourcing and housing, rigorous quality control checks throughout the study, and comprehensive data analysis, all contributing to reliable and comparable results across experiments.
Published Data
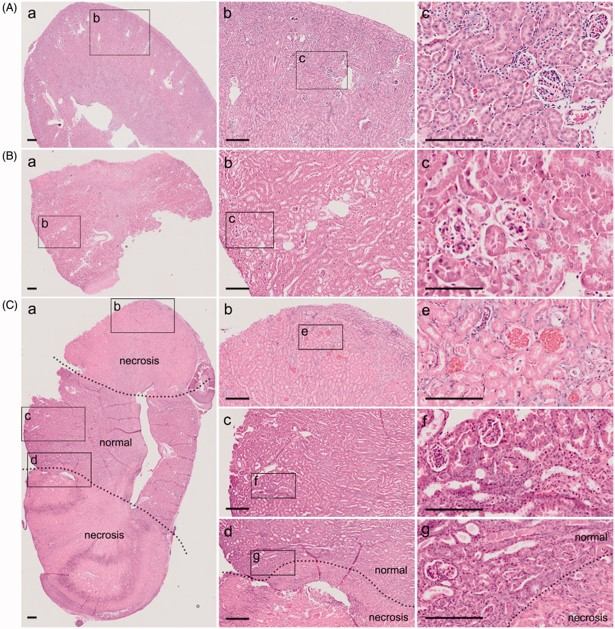 Fig.2 A novel modeling method to simulate conventional 5/6 Nx.2,3
Fig.2 A novel modeling method to simulate conventional 5/6 Nx.2,3
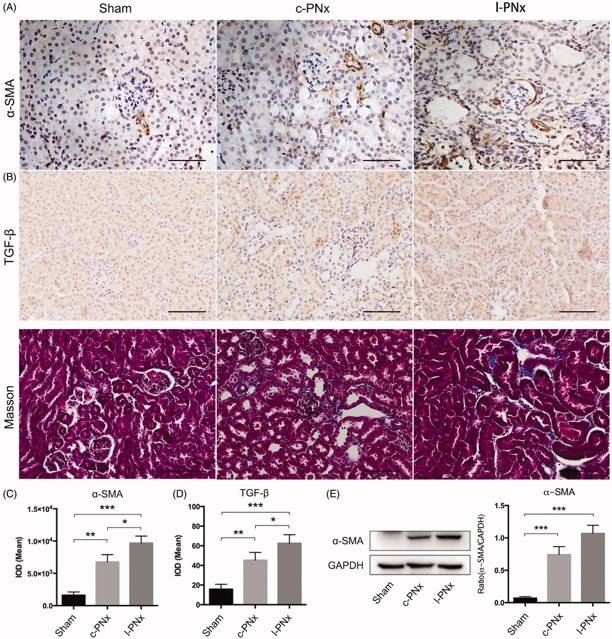 Fig.3 Kidney fibrosis induced by refined 5/6 Nx.2,3
Fig.3 Kidney fibrosis induced by refined 5/6 Nx.2,3
The study demonstrated advancements in the 5/6 Nx modeling method itself. Researchers reported a novel and efficient 5/6 Nx modeling technique that significantly reduced animal mortality and simplified surgical procedures compared to conventional methods. The study confirmed that this optimized model successfully induced robust renal fibrosis, with increased levels than traditional approaches, while offering improved animal welfare and experimental practicality. This highlights the ongoing efforts to refine preclinical models for more ethical and efficient research.
References
- Lenihan, Colin R et al. "Glomerular Function and Structure in Living Donors: Lessons from Single Nephron Studies." Current transplantation reports vol. 3 (2016): 24-32. DOI: 10.1007/s40472-016-0092-y
- Tan, Rui-Zhi et al. "An optimized 5/6 nephrectomy mouse model based on unilateral kidney ligation and its application in renal fibrosis research." Renal failure vol. 41,1 (2019): 555-566. DOI: 10.1080/0886022X.2019.1627220
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
