High-Fat Diet (HFD) induced Heart Failure Modeling & Pharmacodynamics Service
Creative Biolabs stands ready to address this critical need, providing a variety of well-established models to evaluate the efficacy of novel HF therapies.
Introduction
Heart failure (HF) represents a major global health challenge, impacting millions and imposing significant burdens on healthcare systems worldwide. Characterized by the heart's inability to pump sufficient blood to meet the body's demands, HF leads to severe symptoms and poor prognosis. Understanding its complex pathology and developing effective treatments is paramount.
High-Fat Diet-Induced HF Model
The high-fat diet (HFD)-induced HF Model is a cornerstone for investigating the intricate link between metabolic dysfunction and cardiac disease. This model faithfully recapitulates key features of human metabolic syndrome, including obesity, insulin resistance, and dyslipidemia, which are known precursors to various forms of HF, particularly HF with preserved ejection fraction (HFpEF) and diabetic cardiomyopathy.
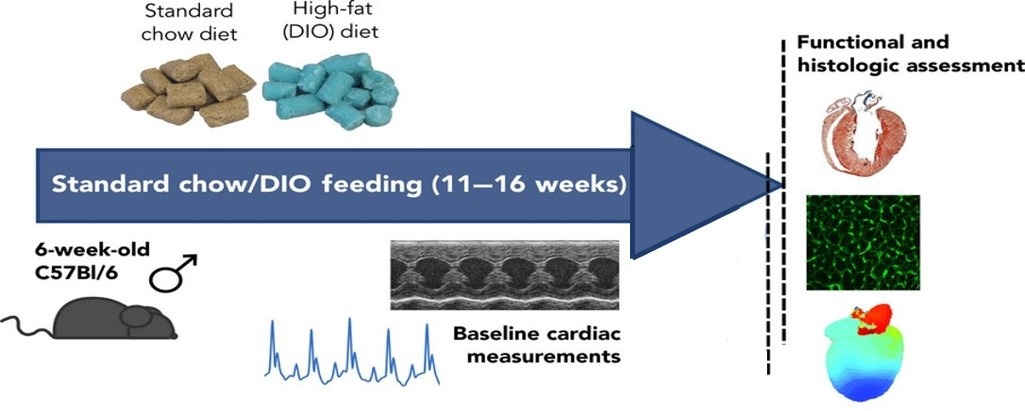 Fig.1 Surgical procedures of the diet-induced obesity (DIO).1
Fig.1 Surgical procedures of the diet-induced obesity (DIO).1
Model Construction Steps
Our meticulous approach ensures the generation of a robust and translationally relevant model, mirroring the progressive nature of the human condition:
01Strain and Species Selection
We begin by selecting genetically appropriate rodent strains, such as C57BL/6 or CD1 mice, known for their susceptibility to metabolic challenges. For broader research applications requiring larger animal models, well-characterized rat strains are also available.
02Dietary Intervention
Animals are placed on a specifically formulated HFD, typically providing 60% of total calories from fat. A control group receives a standard chow diet for comparative analysis.
03Chronic Diet Exposure
The HFD is administered for a prolonged duration, ranging from as short as 20 weeks for early metabolic and cardiac changes, to 12-24 weeks or even up to 11 months for robust cardiac remodeling and overt HF phenotypes.
04Longitudinal Monitoring
Throughout the study, animals undergo regular assessment of systemic metabolic parameters such as body weight, glucose levels, and lipid profiles to track the progression of metabolic dysfunction.
05Cardiac Phenotyping
Concurrently, cardiac function and structural remodeling are longitudinally monitored using advanced imaging techniques like echocardiography, allowing for the detection of subtle and progressive cardiac impairments.
Strengths and Limitations
Strengths:
- Clinical Relevance: Accurately mimics the chronic metabolic stress leading to HF in humans, particularly diabetic cardiomyopathy and HFpEF.
- Progressive Pathology: Allows for the study of disease progression from early metabolic dysfunction to advanced cardiac remodeling and dysfunction.
- Multi-Factorial Etiology: Enables investigation into the synergistic effects of obesity, dyslipidemia, insulin resistance, and inflammation on the heart.
Limitations:
- Extended Duration: Requires a relatively long study duration to induce significant cardiac pathology, which can increase project timelines.
- Resource Intensity: Comprehensive phenotyping and longitudinal monitoring necessitate significant resources and expertise.
- Variability: Model outcomes can exhibit variability depending on specific diet composition, genetic background of the strain, and environmental factors.
Evaluation Platform
Our comprehensive evaluation platform leverages cutting-edge technologies for a holistic understanding of HFD-induced HF:
- Cardiac Function Assessments: Utilizes advanced imaging like echocardiography (ejection fraction, diastolic parameters, strain) and invasive pressure-volume catheterization.
- Structural Remodeling: Evaluated through histopathology (hypertrophy, fibrosis, immune cell infiltration, apoptosis) and electron microscopy.
- Systemic Metabolic Parameters: Comprehensive profiling includes glucose, insulin, lipid panels, adipokines, and AGEs.
- Inflammation & Oxidative Stress Markers: Monitored via inflammatory cell infiltration, key cytokines, macrophage activation, and oxidative stress indicators (MDA, GSH, SOD, CAT).
- Molecular & Biochemical Markers: Involve gene expression (qPCR, RNA-Seq) for pathways like inflammation, metabolism, and cell death; protein expression (Western Blot, ELISA, Proteomics) for signaling and stress markers; and metabolomics for tissue and plasma changes.
Applications
- Simulated Diseases: This model is ideal for simulating and studying the pathogenesis and progression of human diabetic cardiomyopathy, HF with preserved ejection fraction (HFpEF), and other forms of HF linked to metabolic syndrome.
- Drug Evaluation: It serves as a robust platform for evaluating novel pharmacological agents targeting diverse pathways, including those involved in metabolic regulation, inflammation, oxidative stress, cardiac fibrosis (e.g., collagen deposition), cardiomyocyte apoptosis, and immune cell modulation.
- Treatment Assessment: The model is highly effective for assessing the therapeutic potential of various interventions, such as dietary changes (e.g., intermittent fasting strategies), exercise regimens, and nutraceutical compounds aimed at preventing or reversing HFD-induced cardiac dysfunction.
- Biomarker Discovery: Enables the identification and validation of novel diagnostic, prognostic, and predictive biomarkers related to cardiac remodeling, metabolic shifts, myocardial injury, and immune-mediated cardiac inflammation.
Related Heart Failure Models
PA Constriction induced Right HF Model
Ascending Aortic Arch Constriction induced Post-Pressure Overload Heart Failure Model
Abdominal Aortic Stenosis induced Left HF Model
DOCA & Salt induced Left HF Model
AngII induced Chronic Heart Failure Model
ACF induced Anterior Pressure Overload Heart Failure Model
HFHC Diet induced Heart Failure Model
5/6 Nephrectomy induced Heart Failure Model
Pulmonary Hypertension induced Right Heart Failure Model
Renal Artery Constriction induced Hypertensive Heart Failure Model
Our Advantages
- High Reproducibility: Our models consistently deliver reliable and reproducible results.
- Translational Relevance: Models are designed to provide data highly applicable to human conditions.
- Expert Guidance: Our multi-disciplinary team of cardiovascular biologists, pathologists, and metabolic specialists offers unparalleled scientific support.
- Comprehensive Phenotyping: We provide a full spectrum of state-of-the-art phenotyping capabilities.
- Collaborative Approach: We work closely with clients from study design to data interpretation to achieve research goals efficiently.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs provides unparalleled preclinical research services in the field of HF. We are dedicated to accelerating your drug discovery and development efforts. Please do not hesitate to reach out to our scientific team to discuss how our HFD-induced HF model can be tailored to support your specific research needs.
FAQs
-
Q1: How does this HFD model differ from other HF models like TAC?
A: The HFD model simulates HF from chronic metabolic stress, relevant to diabetic cardiomyopathy and HFpEF. TAC, in contrast, induces pressure-overload HF. Our HFD model uniquely captures the complex interplay between systemic metabolic dysfunction and cardiac pathology.
-
Q2: Can diet composition or mouse strain be customized?
A: Yes, we offer flexibility in customizing HFD composition (e.g., fat sources/percentages) to explore specific dietary impacts. Various mouse or rat strains can also be utilized to match genetic predispositions, aligning with your scientific hypothesis.
-
Q3: How does Creative Biolabs ensure data reproducibility and reliability?
A: We maintain stringent quality control, including standardized animal husbandry, precise diet administration, rigorous blinding, and state-of-the-art equipment. Our expert team follows validated protocols and conducts internal reviews, ensuring high data quality and reproducibility.
-
Q4: Is it possible to test combination therapies in this model?
A: Yes, the HFD-induced model is highly suitable for combination therapies. Its multi-factorial etiology allows assessment of interventions targeting various pathways (metabolic, inflammatory, anti-fibrotic) simultaneously or sequentially. Our team helps design complex protocols for understanding synergistic effects.
-
Q5: How do you differentiate between systolic and diastolic dysfunction?
A: We meticulously assess both functions via advanced echocardiography and invasive pressure-volume catheterization. Echocardiography measures EF/FS (systolic) and E/A/E/e' ratios (diastolic). Pressure-volume loops provide gold-standard invasive data like dP/dtmax (systolic) and dP/dtmin/Tau (diastolic) for precise quantification.
Published Data
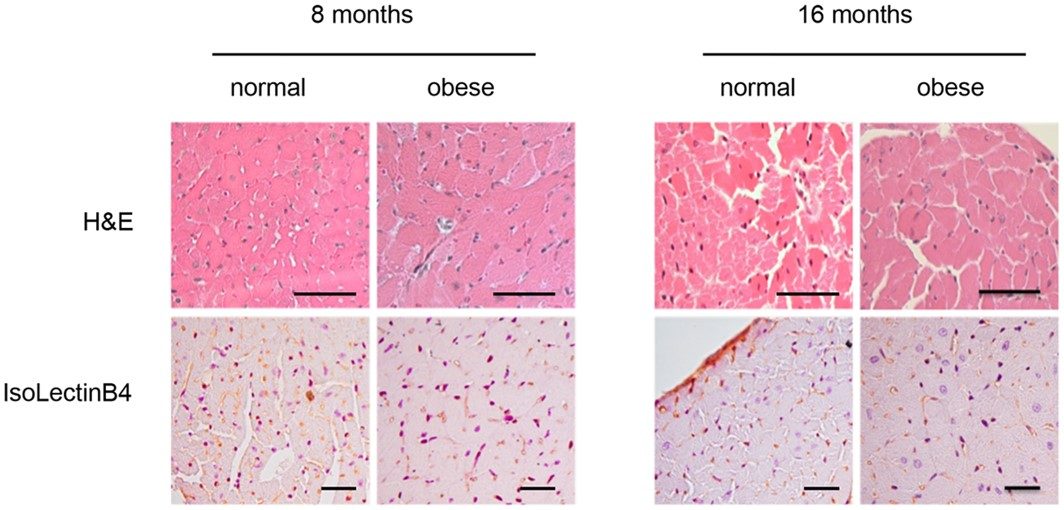 Fig.2 Light microscopy features of cardiac structure of normal and obese mice.2
Fig.2 Light microscopy features of cardiac structure of normal and obese mice.2
A study utilizing a long-term HFD in mice, showed that this model faithfully recapitulates the early phases of human diabetic cardiomyopathy, including the development of hyperglycemia, insulin resistance, cardiac remodeling (increased heart size, thickened cardiac fibers, collagen type I and III overexpression), and the associated diastolic dysfunction. These findings underscore the model's robustness in revealing the etiology, course, and outcomes of metabolically-driven HF, providing a solid foundation for therapeutic discovery.
References
- Holzem, Katherine M et al. "Diet-induced obesity promotes altered remodeling and exacerbated cardiac hypertrophy following pressure overload." Physiological reports vol. 3,8 (2015): e12489. Distributed under Open Access license CC BY 4.0, without modification. The image was modified by extracting and using only part of the original image. https://doi.org/10.14814/phy2.12489
- Calligaris, Sebastián D et al. "Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy." PloS one vol. 8,4 e60931. 11 Apr. 2013. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1371/journal.pone.0060931
For Research Use Only.
