Diethylnitrosamine (DEN) & High-Fat & High-Cholesterol Diet induced Non-Alcoholic Steatohepatitis (NASH) Modeling & Pharmacodynamics Service
Creative Biolabs offers a wide range of well-established preclinical models that enable comprehensive evaluation of NASH drug candidates, providing essential insights into the disease's pathophysiology and therapeutic strategies.
Introduction
Non-alcoholic steatohepatitis (NASH) is a progressive liver disease characterized by the accumulation of fat in the liver, accompanied by inflammation and damage to liver cells. It is a more severe form of non-alcoholic fatty liver disease (NAFLD), which is primarily associated with metabolic risk factors such as obesity, type 2 diabetes, hyperlipidemia, and insulin resistance. NASH can progress to liver fibrosis, cirrhosis, and eventually liver failure or hepatocellular carcinoma (HCC) if left untreated. The pathogenesis of NASH involves complex interactions between metabolic disturbances, oxidative stress, and inflammation, contributing to liver injury and fibrosis. Although early stages of the disease are typically asymptomatic, diagnosis is often confirmed through liver biopsy or advanced imaging techniques. With increasing global prevalence, NASH is emerging as a major public health concern. Currently, there are no FDA-approved drugs specifically for NASH, and treatment is focused on managing risk factors and promoting lifestyle changes. Given the potential for progression to severe liver damage, effective therapies are urgently needed.
Disease Models and Applications
The Diethylnitrosamine (DEN) and High-Fat & High-Cholesterol Diet induced NASH Model is widely used to simulate liver injury and progression to NASH. The model is created by administering DEN, a carcinogen known to induce liver damage, alongside a high-fat and high-cholesterol diet to induce obesity, lipid accumulation, and liver fibrosis. DEN initiates liver cell damage, while the high-fat and high-cholesterol diet promotes the development of metabolic conditions such as hyperlipidemia and insulin resistance, common in NASH. This model closely resembles human NASH in terms of histological features, including hepatocyte ballooning, steatosis, and fibrosis. It is useful for studying the progression of liver damage and testing drug candidates aimed at reducing inflammation and liver fibrosis. However, one limitation of this model is that it relies on DEN, which may not fully replicate the human NASH onset in the absence of carcinogenic factors. Despite this, the model is valuable for evaluating potential therapeutics targeting both early and advanced stages of NASH.
- Simulates: The Diethylnitrosamine (DEN) & High-Fat & High-Cholesterol Diet induced NASH Model simulates the pathogenesis of NASH by combining chemical induced liver damage with diet induced metabolic dysfunction. This model replicates key features of NASH, such as inflammation, fat deposition, and fibrosis, making it suitable for studying disease progression and therapeutic strategies.
- Evaluates Drugs: This model is used to evaluate a range of drug candidates targeting different aspects of NASH, including anti-inflammatory agents, lipid-lowering drugs, and compounds aimed at reversing liver fibrosis. The effectiveness of these drugs can be assessed based on their ability to reduce liver damage, lower serum lipid levels, and modulate liver inflammation and fibrosis.
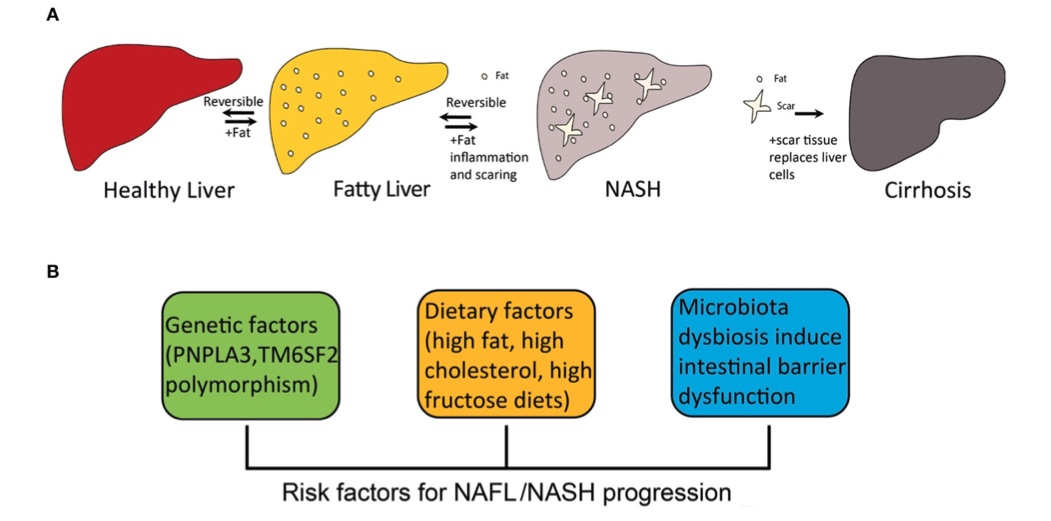 Fig. 1 The progression of fatty liver disease and risk factors for NAFL/NASH progression.1
Fig. 1 The progression of fatty liver disease and risk factors for NAFL/NASH progression.1
Measurements
We offer a variety of measurements for evaluating drug efficacy in the Diethylnitrosamine (DEN) & High-Fat & High-Cholesterol Diet induced NASH Model, utilizing advanced technologies, including but not limited to:
- General observations: body weight, liver size, serum lipid levels, and stool consistency.
- Histological analysis: Hematoxylin and eosin (H&E) staining to evaluate liver architecture, fat accumulation, and inflammation.
- Oil Red O staining: To assess lipid accumulation in liver tissues.
- Immunohistochemistry: Detection of immune cell infiltration (e.g., T-cells, macrophages) in liver tissues.
- Cytokine profiling (e.g., ELISA): Measurement of pro-inflammatory mediators such as TNF-α, IL-6, and IL-1β.
- Hematology and serum biomarkers: Analysis of liver enzymes (e.g., ALT, AST), bilirubin levels, and lipid profiles.
- Gene/protein expression profiling: RT qPCR and Western blotting for key genes and proteins involved in inflammation, lipid metabolism, and fibrosis (e.g., COL1A1, TNF-α, SREBP-1c).
In addition to the DEN and high-fat-high-cholesterol induced NASH model, our expertise extends to creating other specialized animal models for NASH research. Our team can assist with experimental design, model selection, and data analysis to ensure the most effective approach for your research.
Related Services
We provide a full suite of services for preclinical studies, including custom animal model development, drug efficacy testing, pharmacokinetics studies, and histopathological analyses. Our team of experts ensures the design and execution of research projects that are aligned with your scientific goals.
- Diet induced Obesity (DIO) Mouse NASH Model
- High-Fat Diet induced NASH Model
- Methionine Choline-Deficient (MCD) Diet induced NASH Model
- Choline-Deficient L-Amino Acid-Defined (CDAA) Diet induced NASH Model
- High-Fat & High-Carbohydrate Diet induced NASH Model
- High-Fat & High-Cholesterol Diet induced NASH Model
- High-Fat & High-Cholesterol Diet & Fructose induced NASH Model
- High-Fat & Fructose induced NASH Model
- High-Fat & CCL4 induced NASH Model
- Streptozotocin (STZ) & High-Fat induced NASH Model
- MC4R KO Mouse Model
- LDLR KO Mouse Model
Advantages
- Advanced Expertise: Our team comprises specialists with extensive experience in liver disease models and drug testing, ensuring that your research benefits from expert guidance.
- Comprehensive Model Portfolio: We offer a variety of validated preclinical models tailored to NASH, allowing you to select the most suitable one for your specific research needs.
- Flexible Solutions: We adapt to your project's requirements, whether you need model customization, experimental design support, or specific endpoints to evaluate drug efficacy.
- High-Quality Data: Our robust experimental methods and cutting-edge technology deliver accurate, reproducible results that help advance your therapeutic research.
- Global Reputation: Trusted by industry leaders, researchers, and pharmaceutical companies worldwide to deliver high-quality preclinical research services.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What are the key features of NASH?
A: NASH is characterized by liver inflammation, fat accumulation (steatosis), and varying degrees of fibrosis. It can progress to cirrhosis and hepatocellular carcinoma (HCC) if left untreated, and is associated with metabolic conditions like obesity, type 2 diabetes, and hyperlipidemia.
-
Q: How do the preclinical models help in NASH research?
A: Our preclinical models simulate the key features of NASH, such as liver inflammation, steatosis, and fibrosis, making them ideal for studying disease progression and testing potential therapies.
-
Q: What types of drugs can be tested using these models?
A: Our NASH models are used to evaluate a wide range of therapeutic agents, including anti-inflammatory drugs, lipid-lowering therapies, and agents aimed at reducing liver fibrosis.
-
Q: How do we select the right NASH model for our research?
A: Our team will work with you to understand your research goals and select the most appropriate model based on disease progression, drug target, and desired endpoints.
-
Q: Can you provide custom solutions for NASH research?
A: Yes, we offer customized solutions, including model development, experimental design, and data analysis, tailored to the specific needs of your study.
Published Data
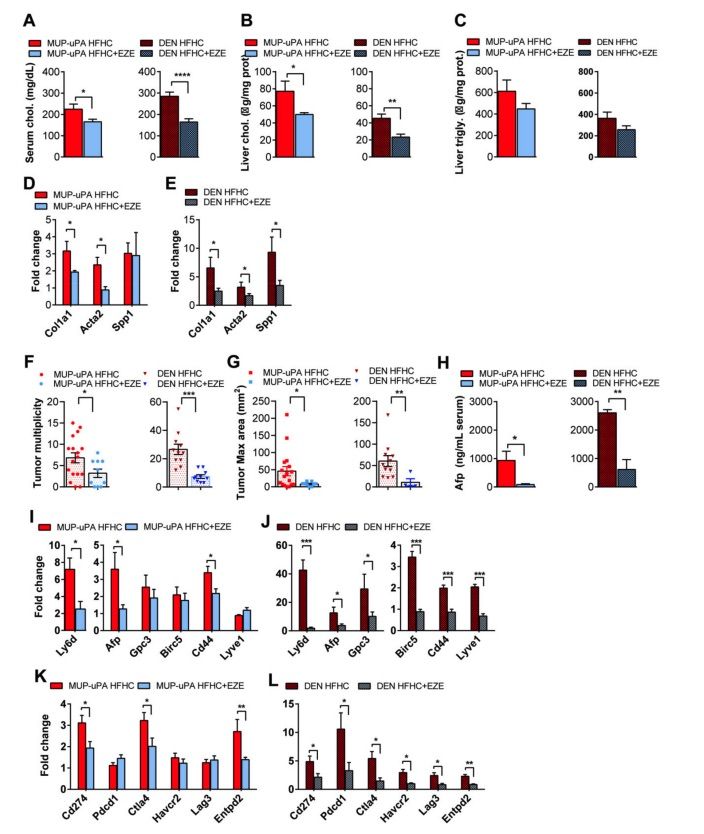 Fig. 2 MUP-uPA or DEN-treated animals fed the HFHC diet supplemented with ezetimibe were compared to their corresponding HFHC-fed group.2
Fig. 2 MUP-uPA or DEN-treated animals fed the HFHC diet supplemented with ezetimibe were compared to their corresponding HFHC-fed group.2
To further investigate the role of cholesterol as a tumor promoter in NASH-driven hepatocellular carcinoma (HCC), the impact of preventing dietary cholesterol absorption on HCC development was tested. MUP-uPA mice and DEN-treated wild-type (WT) mice were fed a high-fat, high-cholesterol (HFHC) diet, either supplemented with ezetimibe, which inhibits cholesterol absorption by targeting Niemann-Pick C1 Like 1 (NPC1L1) in the intestine, or without it. Ezetimibe treatment successfully reduced serum and liver cholesterol levels in both MUP-uPA and DEN-treated WT mice, without affecting liver triglyceride levels (Figure 2A–C). Moreover, ezetimibe ameliorated the HFHC diet induced upregulation of fibrosis-related genes, such as Col1a1, Acta2, and Spp1, in both MUP-uPA and DEN-treated WT mice (Figure 2D, E). Importantly, ezetimibe treatment significantly reduced the multiplicity and maximum area of liver tumors in both mouse models fed the HFHC diet (Figure 2F, G). Notably, DEN-treated WT mice exhibited a higher tumor multiplicity than MUP-uPA mice, although the maximum tumor area was similar between the two groups (Figure 2F, G). Tumor marker expression profiles showed that mRNA levels of Ly6d, Afp, Gpc3, Birc5, and Lyve1 were elevated in DEN-treated WT mice compared to MUP-uPA mice, with Cd44 expression being similar in both models (Figure 2H–J). Furthermore, the upregulation of immune checkpoint markers, including Cd274, Ctla4, and Entpd2, induced by the HFHC diet, was significantly inhibited by ezetimibe treatment in both MUP-uPA and DEN-treated WT mice (Figure 2K, L). Ezetimibe also downregulated the mRNA levels of Pdcd1, Havcr2, and Lag3 in the DEN-treated model, but not significantly in the MUP-uPA mice (Figure 2K, L). These findings suggest that ezetimibe effectively prevents the tumor-promoting role of cholesterol in liver tumorigenesis and NASH-driven HCC.
References
- Zhu, Bo et al. "Non-alcoholic Steatohepatitis Pathogenesis, Diagnosis, and Treatment." Frontiers in cardiovascular medicine vol. 8 742382. 7 Sep. 2021, DOI:10.3389/fcvm.2021.742382. Distributed under an Open Access license CC BY 4.0, without modification.
- Ribas, Vicent et al. "Dietary and Genetic Cholesterol Loading Rather Than Steatosis Promotes Liver Tumorigenesis and NASH-Driven HCC." Cancers vol. 13,16 4091. 13 Aug. 2021, DOI:10.3390/cancers13164091. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
