L-NAME induced Hypertension Modeling & Pharmacodynamics Service
Introduction
Hypertension, a pervasive global health challenge, significantly contributes to cardiovascular morbidity and mortality. Its intricate pathophysiology necessitates sophisticated research models for effective therapeutic development.
At Creative Biolabs, with decades of leadership in preclinical research, we offer a diverse portfolio of meticulously validated hypertension models, empowering our clients to rigorously evaluate the efficacy of novel antihypertensive interventions and accelerate their drug discovery pipelines.
L-NAME-Induced Hypertension Model
The L-NAME (L-NG-Nitro arginine methyl ester)-induced hypertension model is a widely recognized and indispensable tool in cardiovascular research. It operates on the principle of nitric oxide (NO) deficiency, as L-NAME is a non-selective inhibitor of nitric oxide synthase (NOS). By blocking NO production, the model reliably induces sustained vasoconstriction, leading to elevated systemic arterial pressure. This chronic hypertensive state closely mimics key features of human essential hypertension, including endothelial dysfunction, vascular remodeling, and progressive end-organ damage in the heart and kidneys.
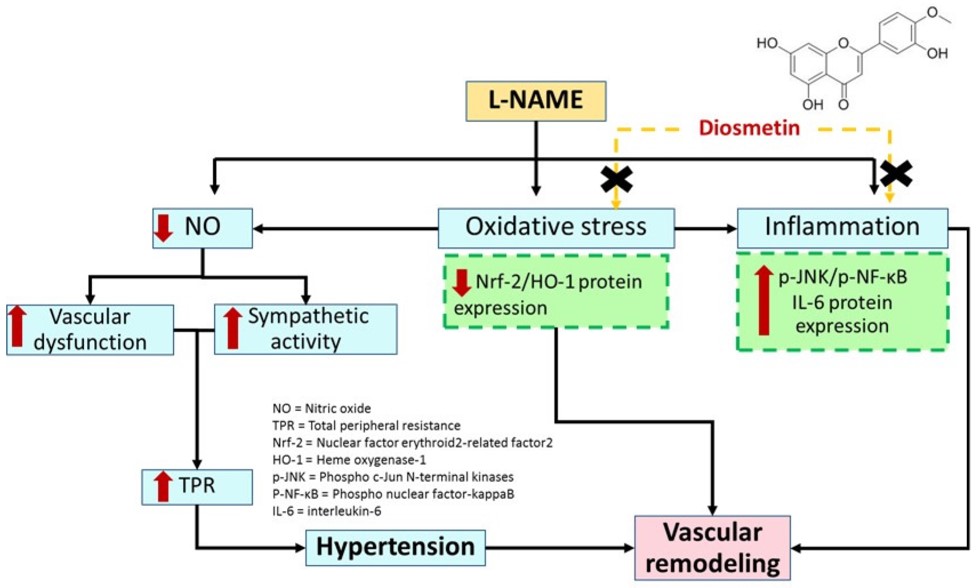 Fig.1 Study of the mechanism of diosmetin in L-NAME-induced hypertensive rats.1,3
Fig.1 Study of the mechanism of diosmetin in L-NAME-induced hypertensive rats.1,3
Model Construction Steps
The construction of the L-NAME-induced hypertension model is a well-established and standardized procedure. The general strategy involves chronic systemic administration of L-NAME to rodents, leading to a stable and reproducible hypertensive phenotype.
01Animal Selection
Healthy rodents, most commonly Wistar rats or specific strains of mice, are selected based on the research objectives.
02L-NAME Administration
L-NAME is typically dissolved in the animals' drinking water, allowing for continuous and consistent exposure. The concentration and duration of L-NAME administration are carefully determined to achieve a stable hypertensive state, usually over several weeks.
03Blood Pressure Monitoring
Throughout the induction period, blood pressure is regularly monitored using non-invasive methods like tail-cuff plethysmography or, for more precise and continuous measurements, invasive radiotelemetry in conscious, unrestrained animals.
04Confirmation of Hypertension
A sustained increase in mean arterial pressure above a predefined threshold confirms successful hypertension induction.
05Endpoint Collection
Following the induction and any intervention period, animals are subjected to comprehensive physiological, biochemical, and histological analyses to assess the severity of hypertension and the effects of interventions.
Strengths and Limitations
Strengths:
- High Reproducibility: Consistently induces stable hypertension, ensuring reliable experimental outcomes.
- Translational Relevance: Mimics key pathological features of human essential hypertension, including endothelial dysfunction, vascular remodeling, and end-organ damage.
- Cost-Effectiveness: Relatively straightforward to implement and maintain compared to some genetically modified or surgical models.
Limitations:
- Non-Specific Effects: L-NAME is a non-selective NOS inhibitor, potentially affecting all NOS isoforms (eNOS, nNOS, iNOS), which might introduce broader physiological changes beyond just vascular tone.
- Species Differences: Responses to L-NAME can vary between different rodent species and strains, requiring careful consideration during study design.
Evaluation Platform
Creative Biolabs' state-of-the-art evaluation platform offers comprehensive insights into the L-NAME model. Our approach integrates diverse methodologies and advanced instrumentation to provide a holistic understanding of disease progression and therapeutic efficacy.
Key Physiological Parameters:
- Blood pressure (measured via telemetry for continuous, unrestrained monitoring or tail-cuff plethysmography)
- Heart rate and ECG
- Vascular reactivity (assessed through ex vivo myography)
- Cardiac function (evaluated using echocardiography and hemodynamic measurements)
- Renal function (including proteinuria and creatinine clearance)
- Cardiovascular reflex sensitivity
Biomarker Analysis:
- Oxidative stress markers (e.g., Malondialdehyde (MDA), Superoxide Dismutase (SOD))
- Inflammatory cytokines (e.g., Tumor Necrosis Factor-alpha (TNF-α), Interleukin-6 (IL-6))
- Fibrosis markers
- Gene and protein expression (e.g., endothelial Nitric Oxide Synthase (eNOS), p47phox)
Applications
- Disease Modeling: Crucial for simulating cardiovascular and renal pathologies like essential hypertension, endothelial dysfunction, and vascular/kidney diseases. It also investigates complex conditions such as preeclampsia and hypertension-related neurological aspects.
- Therapeutic Evaluation: Provides a robust platform for evaluating diverse interventions, including traditional antihypertensive drugs, natural products/plant extracts, specific compounds, and innovative nano-particle delivery systems.
- Mechanistic Research: Beyond drug screening, it is invaluable for dissecting fundamental hypertension mechanisms and complications, exploring roles of oxidative stress, inflammation, the RAAS, sympathetic nervous system activity, and cardiovascular neural reflexes.
Related Hypertension Models
Our Advantages
- Expert Study Design: Tailored to your specific research questions, ensuring optimal experimental parameters.
- Robust Model Execution: Meticulous model induction, characterization, and comprehensive endpoint analysis utilizing cutting-edge facilities.
- Rigorous Quality Control: Adherence to the highest scientific standards for data generation and analysis, providing meticulously interpreted results.
- Deep Translational Insights: Helping you understand the clinical relevance of your findings, accelerating your drug discovery and development.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Leverage Creative Biolabs' unparalleled expertise and comprehensive services in the L-NAME-induced hypertension model to advance your cardiovascular research. We provide reliable, high-quality data and translational insights. Contact us today to discuss your specific project needs and explore how we can support your next breakthrough.
FAQs
-
Q1: How long does it take for hypertension to develop in the L-NAME model?
A: The onset and stability of hypertension in the L-NAME model can vary based on the species, dose, and duration of L-NAME administration. Generally, a sustained hypertensive state is observed within a few days to a few weeks, with chronic administration leading to stable, long-term hypertension suitable for therapeutic evaluations.
-
Q2: What are the key physiological changes observed in this model?
A: Beyond the characteristic elevated blood pressure, the L-NAME model exhibits endothelial dysfunction, significant vascular remodeling, cardiac hypertrophy, and progressive renal damage, such as glomerulosclerosis. Additionally, increased oxidative stress, inflammation, and alterations in cardiovascular neural reflexes are consistently observed, providing a comprehensive disease phenotype.
-
Q3: Can the L-NAME model be used to study preeclampsia?
A: Yes, the L-NAME model is a valuable tool for investigating pregnancy-related hypertension, including preeclampsia and eclampsia. It allows researchers to explore the underlying mechanisms of these complex conditions and evaluate the efficacy of potential therapeutic interventions aimed at mitigating their severity.
-
Q4: What types of endpoints can Creative Biolabs evaluate in L-NAME studies?
A: Creative Biolabs offers comprehensive endpoint analysis, encompassing physiological assessments like telemetry for continuous blood pressure and ECG, and ex vivo vascular reactivity studies using myography. We also perform detailed evaluations of cardiac and renal function, extensive biomarker analysis, histopathology, immunohistochemistry, and molecular biology studies to provide a holistic understanding of treatment effects.
-
Q5: How do you ensure the quality and reproducibility of L-NAME studies?
A: We adhere to the highest scientific standards, implementing rigorous quality control protocols and standardized procedures for every stage of model induction and characterization. Our GLP-compliant facilities, combined with the expertise of our seasoned scientific team and meticulous data analysis, collectively ensure high reproducibility and reliable results for all L-NAME studies.
-
Q6: Is the L-NAME model suitable for evaluating natural products or plant extracts?
A: Absolutely. The L-NAME model is widely utilized for screening the antihypertensive, cardioprotective, and nephroprotective effects of various natural products and plant extracts. It provides a robust and translatable platform to investigate their mechanisms of action and assess their therapeutic potential in a preclinical setting.
Published Data
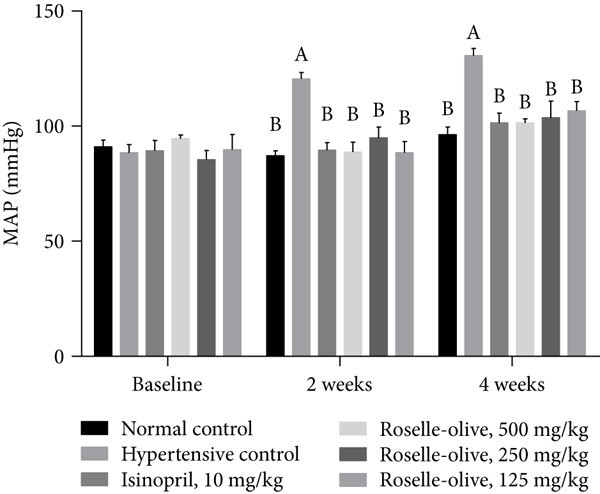 Fig.2 Effect of Roselle-Olive combination on the mean arterial blood pressure (MAP) in L-NAME-induced hypertensive rats.2,3
Fig.2 Effect of Roselle-Olive combination on the mean arterial blood pressure (MAP) in L-NAME-induced hypertensive rats.2,3
The study utilized the L-NAME-induced hypertension model to evaluate the therapeutic potential of a Roselle-Olive combination. The research demonstrated that this combination exhibited significant antihypertensive effects and provided protection against end-organ damage in L-NAME-induced hypertensive rats. This case exemplifies the model's effectiveness in assessing the efficacy of natural product formulations and functional foods, underscoring its relevance for preclinical development in the nutraceutical and pharmaceutical industries.
References
- Meephat, Sariya et al. "Diosmetin Ameliorates Vascular Dysfunction and Remodeling by Modulation of Nrf2/HO-1 and p-JNK/p-NF-κB Expression in Hypertensive Rats." Antioxidants (Basel, Switzerland) vol. 10,9 1487. 17 Sep. 2021. https://doi.org/10.3390/antiox10091487
- Abdel-Rahman, Rehab F et al. "Antihypertensive Effects of Roselle-Olive Combination in L-NAME-Induced Hypertensive Rats." Oxidative medicine and cellular longevity vol. 2017 (2017): 9460653. https://doi.org/10.1155/2017/9460653
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
