Hyperuricemia Modeling & Pharmacodynamics Services
Creative Biolabs offers a variety of well-established animal models for hyperuricemia, enabling the preclinical evaluation of urate-lowering drugs, anti-inflammatory agents, and kidney protection therapies. Our team provides expert support throughout your research to ensure reliable, high-quality results.
Introduction
Hyperuricemia is a metabolic disorder characterized by elevated levels of uric acid in the bloodstream, which can lead to the formation of urate crystals. These crystals can accumulate in joints, causing gout, or in the kidneys, leading to uric acid nephropathy. Uric acid is primarily produced by the breakdown of purines, substances found in certain foods and beverages, as well as in body tissues. It can occur due to overproduction of uric acid or underexcretion by the kidneys. Common causes include excessive intake of purine-rich foods (e.g., red meat, seafood, alcohol), obesity, kidney dysfunction, and genetic factors. It is often associated with conditions such as gout, kidney stones, hypertension, and cardiovascular diseases.
Hyperuricemia Models
Creative Biolabs offers a comprehensive range of well-established rodent models for hyperuricemia, including the Diet-Induced Hyperuricemia Model, which is induced by high-purine or high-fructose diets. These models are specifically designed to mimic the key aspects of human hyperuricemia, including uric acid overproduction and impaired renal clearance. By replicating dietary-induced increases in uric acid levels, the model provides a valuable platform for evaluating urate-lowering therapies, anti-inflammatory agents, and other related treatments. The Diet-Induced Hyperuricemia Model closely simulates conditions such as gout and uric acid nephropathy, allowing for a detailed investigation of therapeutic candidates aimed at managing uric acid levels and preventing crystal deposition in joints and kidneys. Our team of experts will support your research throughout the project, ensuring high-quality results and comprehensive data analysis during the preclinical phase.
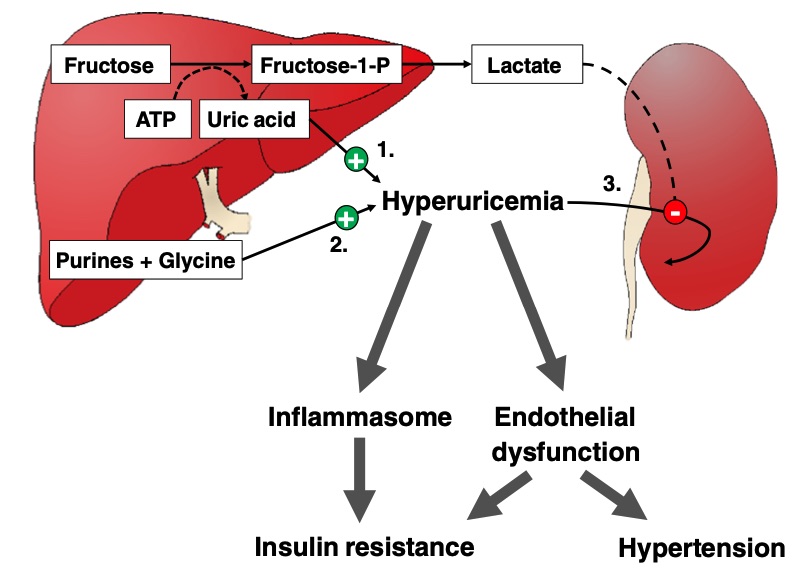 Fig. 1 Potential deleterious effects of fructose-induced hyperuricemia.1,3
Fig. 1 Potential deleterious effects of fructose-induced hyperuricemia.1,3
| Hyperuricemia Model | Simulates | Evaluates Drugs | Animal species |
| Diet-Induced Hyperuricemia Model | Uric acid overproduction due to a high-purine or high-fructose diet | Uric acid-lowering drugs, anti-inflammatory agents, gout therapies, and nephroprotective drugs | Rat |
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in rodent hyperuricemia models, utilizing advanced technologies, including but not limited to:- General Observations: Body weight, mortality rate, urine output, and clinical signs of gout or urate crystal deposition.
- Histopathological Analysis: Kidney and joint tissue damage, urate crystal deposition, and inflammation.
- Immunohistochemistry: Infiltration of immune cells (e.g., macrophages, neutrophils) in joint and renal tissues.
- Cytokine Profiling (e.g., ELISA): Expression levels of inflammatory mediators such as TNF-α, IL-1β, and IL-6.
- Hematology Analysis and Serum Biomarkers: Serum uric acid levels, renal function markers (e.g., BUN, creatinine), and markers of inflammation (e.g., C-reactive protein).
- Gene/Protein Expression Profiling via RT-qPCR and Western Blot Techniques: Expression of urate transporters (e.g., URAT1), inflammatory cytokines, and crystal-induced damage markers (e.g., NLRP3 inflammasome).
In addition to the well-established models, we can develop customized animal models tailored to your specific research needs. Our scientific team is available to assist in experimental design, model selection, and data interpretation to ensure the most effective approach for your hyperuricemia drug evaluation.
Related Services
In addition to our hyperuricemia models, we offer a range of other specialized rodent models for various kidney and urinary system conditions. These models facilitate the testing of therapeutic candidates for a broader spectrum of renal diseases, ensuring a comprehensive approach to evaluating potential treatments. Here's an overview of these models:
- Autoimmune Nephropathy Models
- Acute Kidney Injury (AKI) Models
- Chronic Kidney Disease (CKD) Models
- Metabolic Nephropathy Models
- Kidney Transplantation Models
- Cystitis Models
Our advantages
- Established Expertise: We offer a comprehensive selection of well-characterized animal models tailored for metabolic nephropathy research, with a track record of delivering high-quality results.
- Customized Solutions: Our team works closely with you to tailor experimental design and model selection, ensuring that the models fit the specific needs of your research.
- State-of-the-Art Technologies: We utilize the latest technologies in genomics, proteomics, imaging, and histology to provide detailed and accurate assessments of drug efficacy.
- Scientific Support: Our team of experienced scientists provides full support throughout the project, from planning and implementation to data analysis and interpretation.
- Flexibility and Reliability: We offer fast turnaround times and flexible options for model testing, ensuring that you receive reliable and timely results to advance your research.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What models do you offer for metabolic nephropathy?
We offer models for obesity-related glomerulopathy, diabetic nephropathy, and HDF-CHOL & Salt Feed & 5/6 nephrectomy-induced nephropathy. These models simulate key aspects of metabolic nephropathy, including obesity-related kidney damage, diabetes-induced glomerulosclerosis, and combined metabolic syndrome with renal injury.
-
2. How do your models evaluate drug efficacy?
Our models allow for the assessment of renal function, inflammation, fibrosis, and kidney damage. Measurements include general health status, histopathological analysis, biomarker levels, gene/protein expression, and urine/serum analysis to evaluate therapeutic efficacy.
-
3. Can you assist with experimental design and data analysis?
Yes, our team provides comprehensive support from experimental design to data interpretation. We work closely with you to ensure that the models and assays are tailored to your research goals.
-
4. Are your models validated for preclinical research?
Yes, all of our models are well-established, validated, and widely used in the scientific community. They are designed to closely mimic human metabolic nephropathy and provide reliable preclinical data.
-
5. How long does it take to receive results?
The timeline depends on the complexity of the study and the specific tests requested. We aim to provide reliable results in a timely manner, typically within a few weeks to months, depending on the project scope.
Published Data
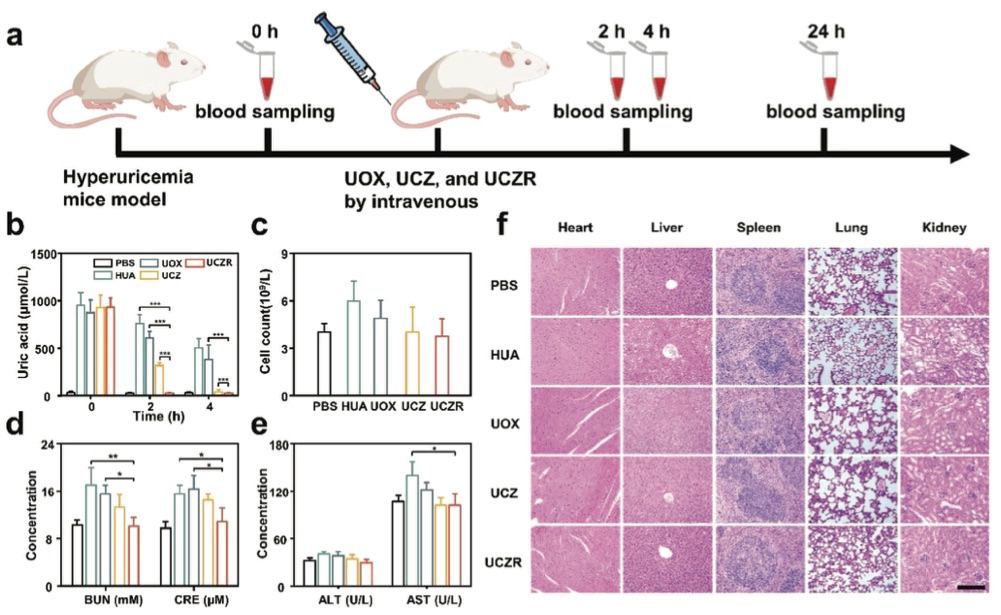 Fig. 2 Schematic illustration of hyperuricemia mice model establishment and treatment process.2,3
Fig. 2 Schematic illustration of hyperuricemia mice model establishment and treatment process.2,3
A mouse model of hyperuricemia was established to evaluate the in vivo efficacy of UCZR in degrading uric acid (UA). Hyperuricemia was induced through subcutaneous injections of oxonic acid potassium (100 mg/kg) and intraperitoneal injections of hypoxanthine (200 mg/kg). The mice were then randomly divided into four groups and treated with PBS, UOX, UCZ, or UCZR (25 U/kg). Plasma UA concentrations were measured at regular intervals, with healthy mice as controls. As shown in Figure 2a, UCZR-treated mice exhibited a rapid decrease in UA levels to normal levels within 2 hours, while PBS-treated mice maintained elevated UA levels. Mice treated with UOX and UCZ showed higher UA levels than UCZR-treated mice (Figure 2b), indicating the superior UA-lowering potential of UCZR. The in vivo toxicity of UCZR was thoroughly assessed through blood biochemistry and histopathological analysis. As presented in Figure 2c, white blood cell (WBC) counts were significantly lower in UCZR-treated mice compared to UOX-treated mice, suggesting minimal immune response. Hepatotoxicity and nephrotoxicity were evaluated by measuring alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine (CRE). As shown in Figure 2d, the levels of ALT and AST in UCZR-treated mice were comparable to those of healthy mice, and the levels of BUN and CRE exhibited similar trends (Figure 2e), suggesting minimal liver and kidney damage. Histological analysis of heart, liver, spleen, lung, and kidney tissues revealed no signs of abnormal cell morphology or inflammation in UCZR-treated mice, as shown in Figure 2f, while UOX-treated mice exhibited severe tissue damage, such as hepatocyte injury and dilatation of renal tubules and glomeruli. These findings confirm that UCZR has minimal toxic effects and demonstrate its potential for safe and effective treatment of hyperuricemia.
References
- Rosset, Robin et al. "Pathogenesis of Cardiovascular and Metabolic Diseases: Are Fructose-Containing Sugars More Involved Than Other Dietary Calories?" Current Hypertension Reports vol. 18,6 (2016): 44. https://doi.org/10.1007/s11906-016-0652-7
- Li, Zeyu et al. "Synthetic Biohybrids of Red Blood Cells and Cascaded-Enzymes@ Metal-Organic Frameworks for Hyperuricemia Treatment." Advanced Science (Weinheim, Baden-Wurttemberg, Germany) vol. 11,5 (2024): e2305126. https://doi.org/10.1002/advs.202305126
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
