Lipopolysaccharide (LPS) induced Disseminated Intravascular Coagulation (DIC) Modeling & Pharmacodynamics Service
Introduction
Disseminated intravascular coagulation (DIC) is a severe, acquired syndrome characterized by systemic activation of the coagulation cascade, leading to widespread microthrombosis and paradoxical bleeding. Often triggered by underlying conditions such as severe infection (sepsis), trauma, or malignancy, DIC can rapidly progress to multi-organ dysfunction and significantly increase mortality.
At Creative Biolabs, we leverage decades of experience to provide a diverse array of meticulously validated DIC models, enabling comprehensive evaluation of novel therapeutic strategies.
Lipopolysaccharide (LPS)-Induced DIC Model
The lipopolysaccharide (LPS)-induced DIC model is a cornerstone in preclinical research, particularly for investigating sepsis-associated coagulopathy. LPS, a potent endotoxin from Gram-negative bacteria, reliably triggers a robust systemic inflammatory and procoagulant response in experimental animals. It closely mimics the "coagulation-dominant" or "fibrinolysis shutdown" phenotype observed in clinical sepsis.
Model Construction Steps
The construction of the LPS-induced DIC model typically involves the systemic administration of LPS to induce a controlled yet severe coagulopathic state. Our standard protocol, based on established methodologies, ensures reproducibility and relevance.
01Animal Preparation
Male Wistar rats, weighing 160-170 g, are acclimatized for at least 3 days in a controlled environment. Animals are fasted for 12 hours prior to the experiment.
02Anesthesia
Prior to LPS administration, animals are anesthetized using anesthetics (30 mg/kg intraperitoneally).
03LPS Administration
A sustained infusion of Escherichia coli 055:B5 LPS (30 mg/kg) diluted in 10 ml physiological saline is administered via the tail vein over a 4-hour period.
04Blood Sampling
Blood samples are collected from the abdominal aorta at various time points (e.g., 0, 1, 3, 4, 5, 7, 9, 11, and 28 hours post-infusion initiation) to monitor the progression of DIC.
05Sacrifice and Tissue Collection
Each rat is humanely sacrificed immediately after blood sampling under deep anesthesia, followed by the collection of target organs (e.g., kidney, liver) for pathological examination.
Strengths and Limitations
Strengths:
- High Clinical Relevance: Closely mimics sepsis-associated DIC, a common and severe form of the syndrome.
- Robust Response: Reliably induces a well-characterized inflammatory and procoagulant state.
- Fibrinolysis Shutdown: Effectively models the critical "fibrinolysis shutdown" phenomenon seen in septic DIC, leading to persistent microthrombosis.
- Organ Damage: Reproduces significant multi-organ dysfunction, allowing for evaluation of organ-protective therapies.
- Established Protocol: Benefits from extensive historical data and standardized methodologies.
Limitations:
- Acute Nature: Primarily models acute DIC and may not fully capture chronic or sub-clinical forms.
- Etiological Specificity: While excellent for sepsis-related DIC, it may not perfectly represent DIC induced by other triggers (e.g., trauma, malignancy).
Evaluation Platform
Our comprehensive evaluation platform integrates advanced biochemical, molecular, cellular, and histopathological analyses, complemented by functional assessments. This multi-faceted approach provides a holistic understanding of disease progression and therapeutic impact.
Key Test Indicators:
- Coagulation Parameters: Prothrombin Time (PT), Activated Partial Thromboplastin Time (aPTT), Fibrinogen levels, Thrombin-Antithrombin Complex (TAT), D-dimer.
- Inflammatory Markers: Cytokine profiling (e.g., TNF-α, IL-1β, IL-6, IL-10, IFN-γ), PAI-1 activity.
- Organ Function Assessment: Plasma creatinine (renal), Alanine Aminotransferase (ALT) (hepatic).
- Histopathology: Glomerular fibrin deposition (% GFD), microthrombosis, tissue injury in target organs (e.g., kidney, liver, lung).
- Survival Analysis: Overall survival rates.
Applications
- Disease Simulation: This model effectively simulates sepsis-associated DIC, faithfully replicating key features like systemic inflammation, widespread microthrombosis, and subsequent organ dysfunction. It also serves as a valuable platform for studying other inflammatory coagulopathies where endotoxin plays a significant pathogenic role.
- Drug Evaluation: It is an ideal system for assessing the efficacy of a wide range of therapeutic agents. This includes novel anticoagulants, anti-inflammatory agents to dampen the cytokine storm, immunomodulators, and therapies targeting coagulation factors or the fibrinolytic system to restore hemostatic balance.
- Therapeutic Strategies: The model is extensively used to evaluate interventions preventing detrimental microvascular thrombosis, thereby reducing severe organ damage. It also assesses strategies to restore anticoagulant balance, mitigate bleeding complications, and ultimately improve patient survival in critical septic conditions.
- Biomarker Discovery: By providing a controlled environment, this model facilitates the identification and rigorous validation of new diagnostic and prognostic biomarkers. These are essential for early detection, monitoring disease severity, and predicting patient outcomes in clinical settings.
Our Advantages
- Unparalleled Scientific Insight: Our team of expert biologists brings deep scientific understanding to every project.
- Validated Models: Access comprehensive, meticulously validated LPS-induced DIC models across various species.
- State-of-the-Art Analytical Readouts: Utilize advanced analytical capabilities for precise and reliable data generation.
- Accelerated Drug Development: Our focus on high-quality, translational data is designed to accelerate your lead generation and drug development pipeline.
- Highest Standards: All studies adhere to stringent regulatory compliance and animal welfare guidelines.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is dedicated to advancing your research in complex coagulopathies. By providing robust LPS-induced DIC models and comprehensive evaluation services, we empower you to develop impactful therapies. Contact us today to discuss how our expertise can support your next breakthrough.
FAQs
-
Q1: How do you ensure the reproducibility and consistency of its LPS-induced DIC model?
A: We maintain stringent quality control measures to ensure high reproducibility. This includes using standardized LPS preparations, precise administration protocols, consistent animal strains and housing conditions, and experienced personnel. Our detailed standard operating procedures (SOPs) and robust data collection methods contribute to the reliability and consistency of our model outcomes.
-
Q2: What are the typical timeframes for observing DIC progression and therapeutic effects in the LPS model?
A: DIC progression in the LPS model can be rapid, with initial coagulation activation occurring within hours of LPS administration. Organ dysfunction and mortality typically become more pronounced after 9 hours and can extend up to 28 hours or longer, depending on the LPS dose and experimental design. Therapeutic interventions are often initiated before or shortly after LPS administration to assess their preventive or ameliorative effects.
-
Q3: How do you handle potential variability in animal responses to LPS?
A: Biological variability is inherent in animal models. We mitigate this through careful animal selection, proper randomization, adequate group sizes, and rigorous statistical analysis. Our experienced team closely monitors animal health and responses, ensuring that any outliers are identified and managed according to ethical guidelines and study protocols.
-
Q4: Is it possible to combine the LPS-induced DIC model with other disease models, such as sepsis models, to create more complex scenarios?
A: Yes, the LPS-induced DIC model is often integrated into broader sepsis models to study the full spectrum of sepsis-associated complications. For instance, combining LPS with other inflammatory stimuli or using models that mimic polymicrobial infection can create more complex and clinically relevant scenarios for evaluating multi-target therapies.
-
Q5: Can you assist with custom study designs or specific research questions related to LPS-induced DIC?
A: Absolutely. We pride ourselves on our collaborative approach. Our scientific team works closely with clients to understand their unique research objectives and design bespoke study protocols. Whether you have a specific therapeutic candidate, a novel mechanism to explore, or a complex research question, we can tailor our services to meet your exact needs.
Published Data
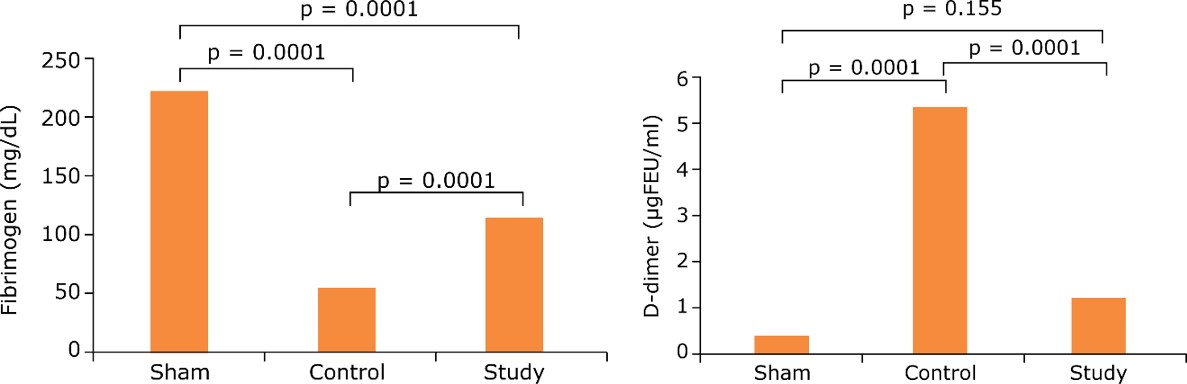 Fig.1 Effect of APC on fibrinogen and D-dimer levels in the LPS-induced DIC model.1
Fig.1 Effect of APC on fibrinogen and D-dimer levels in the LPS-induced DIC model.1
The study investigated the effect of activated protein C (APC) in an LPS-induced DIC rat model. This research highlighted how APC effectively prevented hypercoagulation and consumption coagulopathy by correcting hematological parameters, such as fibrinogen and D-dimer levels, despite prolonging coagulation time. Such findings underscore the model's utility in evaluating potential therapeutic interventions for DIC.
Reference
- Öahin, Abdullah, and Nazmi Özer. "The effect of activated protein C in the experimental disseminated intravascular coagulation model formed by lipopolysaccharide infusion." Acta cirurgica brasileira vol. 35,11 e351102. 18 Dec. 2020. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1590/ACB351102
For Research Use Only.
