- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
Disseminated Intravascular Coagulation Modeling & Pharmacodynamics Services
Introduction
Disseminated intravascular coagulation (DIC) is a severe, life-threatening syndrome characterized by systemic activation of blood coagulation, leading to widespread microthrombi formation and simultaneous consumption of clotting factors, resulting in both thrombotic and hemorrhagic complications. It arises from various underlying conditions such as sepsis, trauma, and cancer, often culminating in multiple organ failure.
At Creative Biolabs, we offer a comprehensive suite of well-established DIC models, enabling precise evaluation of therapeutic candidates and a deeper understanding of this complex disease.
Available Disseminated Intravascular Coagulation Model at Creative Biolabs
The development and application of robust DIC models are paramount for dissecting the intricate pathophysiology of this syndrome, identifying novel biomarkers, and rigorously assessing potential therapeutic interventions. These models allow researchers to investigate specific pathways involved in DIC initiation and progression, from tissue factor expression to fibrinolytic dysregulation. Model construction strategies at Creative Biolabs are meticulously designed to mimic the diverse clinical presentations of human DIC, ensuring high translational relevance for drug discovery.
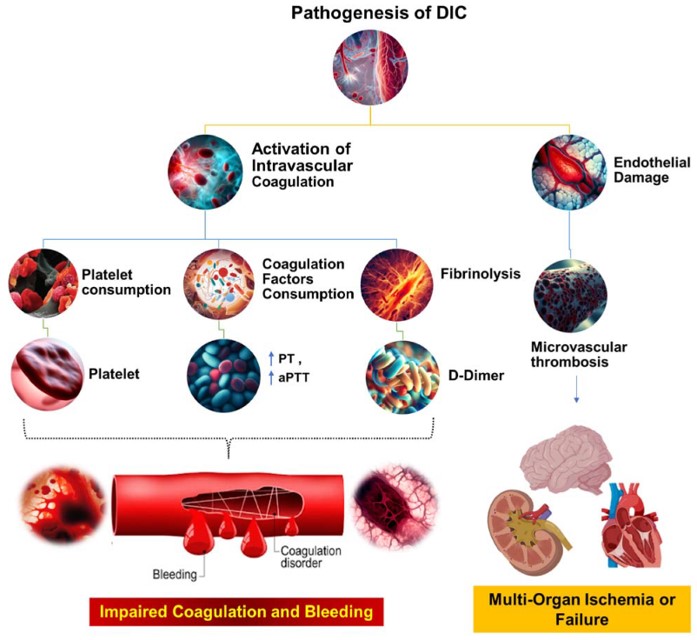 Fig.1 The pathophysiological mechanisms underlying DIC.1,3
Fig.1 The pathophysiological mechanisms underlying DIC.1,3
Our team provides the following rodent Creative Biolabs model, meticulously developed to support your preclinical research needs.
Lipopolysaccharide (LPS) induced DIC Model - This model simulates severe septic DIC via sustained intravenous LPS infusion. It triggers robust inflammation, tissue factor expression, cytokine release, and systemic coagulation. Characterized by suppressed fibrinolysis, sustained fibrin deposition, and significant organ dysfunction, our strategy involves careful LPS dose titration and continuous monitoring of hemostatic parameters and organ function, ensuring reproducible, clinically relevant outcomes.
Evaluation Platform
Our state-of-the-art evaluation platform integrates diverse analytical techniques to provide comprehensive insights into DIC pathophysiology and therapeutic efficacy.
- Biochemical & Molecular Analysis: Platelet Count, Fibrinogen Levels, Thrombin-Antithrombin Complex (TAT), Antithrombin III (AT III) Activity, D-dimer Levels, Plasminogen Activator Inhibitor (PAI) Activity, Cytokine Profiling (e.g., TNF-α、IL-1β、IL-6), and Gene Expression Analysis (e.g., tissue factor, inflammatory mediators).
- Cellular Assays: Assess endothelial cell activation and integrity, monocyte/macrophage tissue factor expression, and platelet aggregation.
- Histopathological Examination: Quantify glomerular fibrin deposition (GFD), microthrombi in various organs, and organ damage scoring (e.g., kidney, liver, lung).
- Organ Function Assessment: Measure Plasma Creatinine (renal function) and Alanine Aminotransferase (ALT) (liver function).
- Imaging Techniques: Utilize microcirculation imaging (e.g., intravital microscopy).
Applications
Disease Simulation: Our models accurately simulate various clinical manifestations of DIC, including sepsis-induced DIC, trauma/hemorrhage-induced DIC, and cancer-associated DIC, as well as aspects seen in acute leukemia and obstetric diseases. This allows for targeted research into specific disease etiologies.
Drug Evaluation: We facilitate the rigorous evaluation of a wide array of therapeutic agents, including classic anticoagulants (e.g., heparin), anti-inflammatory compounds, factor concentrates (e.g., antithrombin, activated protein C, thrombomodulin), direct thrombin inhibitors, factor Xa inhibitors, and platelet-activating factor (PAF) antagonists.
Therapeutic Insights: These models are instrumental for dissecting disease mechanisms, validating novel drug targets, assessing preclinical efficacy and safety profiles, and correlating changes in circulating biomarkers with disease progression and treatment response.
Related Cardiovascular Models
Our Advantages
- Diverse Animal Species: We offer a wide range of animal models, including various rodent strains, allowing for optimal model selection based on your research needs.
- Integrated Evaluation: Our capabilities span comprehensive in vitro (cell-based, microfluidic systems) and in vivo evaluations, providing a holistic understanding of disease and drug effects.
- Expert Team & Quality Systems: With decades of experience, our professional team of biologists and pharmacologists, coupled with a perfect management system, ensures high-quality, reproducible, and regulatory-compliant data.
Work with Us
Inquiry Stage
Project Start
- We provide a detailed project plan, including the required sample quantities, methods and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
Project Progress
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
Project Completion
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
After-Sales Support
- Data storage and archiving.
Contact Us
Leverage Creative Biolabs' extensive experience and scientific acumen in DIC modeling to accelerate your therapeutic discovery programs. Our commitment to cutting-edge research can help bring your innovative solutions closer to patients. Contact us today to discuss your specific project requirements.
FAQs
-
Q1: How do you ensure the clinical relevance of its DIC animal models?
A: We meticulously select and develop models that closely mimic human DIC pathophysiology, considering factors like the inducing agent, dosing regimen, and observation period. Our expertise ensures that the chosen model accurately reflects the specific clinical scenario you aim to investigate, maximizing translational potential.
-
Q2: What specific hemostatic parameters are routinely measured in your DIC models?
A: Our standard panel includes platelet counts, fibrinogen levels, TAT, AT III activity, D-dimer levels, and PAI activity. We also offer more specialized assays based on project requirements.
-
Q3: Can your models differentiate between procoagulant and antifibrinolytic effects of a therapeutic agent?
A: Absolutely. By employing a comprehensive suite of coagulation and fibrinolysis assays, including D-dimer and PAI activity, we can precisely determine whether your compound primarily targets coagulation, fibrinolysis, or both, providing nuanced insights into its mechanism of action.
-
Q4: Is it possible to evaluate the long-term effects of a therapeutic intervention in your DIC models?
A: Yes, our experimental designs allow for extended observation periods to assess the long-term impact of interventions on organ recovery, sustained hemostatic balance, and overall survival, which is crucial for understanding chronic DIC or delayed therapeutic benefits.
-
Q5: What measures are taken to ensure the reproducibility of your DIC models?
A: We adhere to stringent standardization protocols, including careful characterization of inducing agents, consistent administration routes and doses, and standardized animal care. Our experienced team follows rigorous experimental procedures to minimize variability and ensure reliable results.
-
Q6: Can your platform support the identification and validation of novel DIC biomarkers?
A: Indeed. Our comprehensive evaluation platform, including molecular and proteomic analyses, is well-suited for biomarker discovery. We can help identify potential diagnostic, prognostic, or pharmacodynamic biomarkers and then validate them within our established DIC models.
Published Data
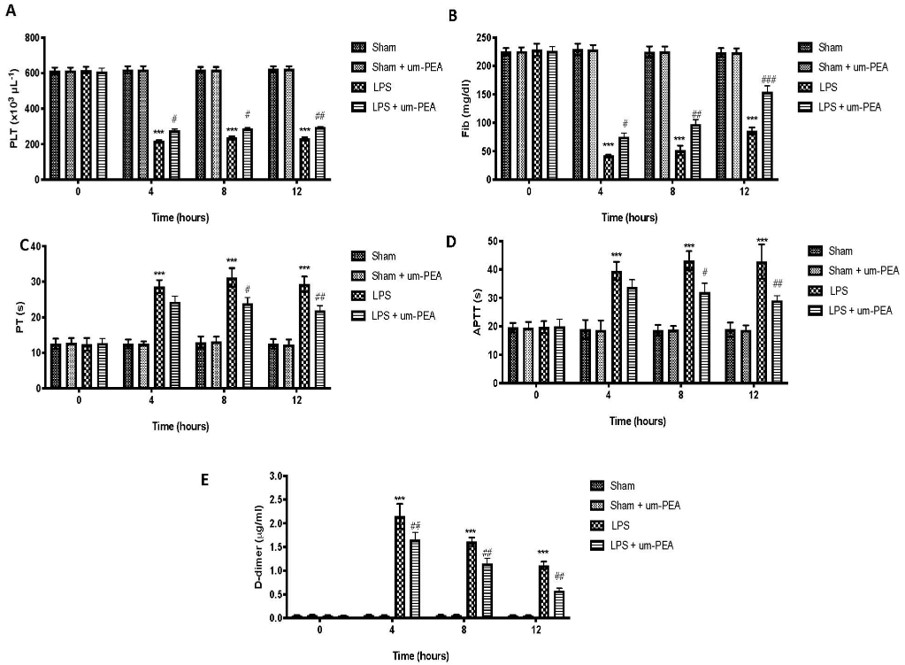 Fig.2 Effect of um-PEA on Blood Coagulation Parameters in LPS-induced DIC model.2,3
Fig.2 Effect of um-PEA on Blood Coagulation Parameters in LPS-induced DIC model.2,3
This research demonstrates how ultramicronized palmitoylethanolamide (um-PEA) effectively mitigated LPS-induced DIC in rats. The project results showed that um-PEA reduced alterations in coagulation markers, decreased proinflammatory cytokine release in plasma and lung, and prevented fibrin deposition and lung damage, underscoring its potential in managing sepsis-induced coagulopathy.
References
- Unar, Ahsanullah et al. "Pathophysiology of Disseminated Intravascular Coagulation in Sepsis: A Clinically Focused Overview." Cells vol. 12,17 2120. 22 Aug. 2023, DOI:10.3390/cells12172120.
- D'Amico, Ramona et al. "Ultramicronized Palmitoylethanolamide in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation." International journal of molecular sciences vol. 22,21 11388. 21 Oct. 2021, DOI:10.3390/ijms222111388.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
