DOCA & Salt induced Left Heart Failure Modeling & Pharmacodynamics Service
At Creative Biolabs, our comprehensive expertise spans a variety of meticulously validated in vivo models, empowering researchers to precisely evaluate the efficacy of novel interventions against the complexities of HF.
Introduction
Heart failure (HF) represents a critical global health challenge, characterized by the heart's inability to pump blood efficiently, leading to systemic dysfunction. Often progressing from chronic conditions like hypertension, HF necessitates robust preclinical models for effective therapeutic development.
DOCA & Salt-Induced Left HF Model
The deoxycorticosterone acetate (DOCA) & Salt model in rodents (typically rats) is a widely utilized and highly relevant experimental paradigm for studying systemic hypertension and its progression to HF, particularly focusing on mineralocorticoid excess. This model effectively recapitulates key pathophysiological features observed in human hypertensive heart disease and chronic kidney disease.
Model Construction Steps
The construction of the DOCA & Salt model involves a precise, multi-step surgical and pharmacological approach:
01Unilateral Nephrectomy
Initially, one kidney is surgically removed (uninephrectomy). This procedure exacerbates the effects of subsequent salt loading by reducing the kidney's capacity to excrete sodium.
02DOCA Administration
Following nephrectomy, animals receive chronic administration of DOCA, a potent synthetic mineralocorticoid. DOCA is typically administered via subcutaneous injections or osmotic minipumps for sustained release.
03High-Salt Diet
Concurrently with DOCA administration, the animals are provided with a high-salt diet, usually 0.9-1% sodium chloride in their drinking water. This high salt intake synergizes with DOCA to induce volume expansion and robust hypertension.
04Monitoring and Progression
Animals are closely monitored for the development of hypertension, cardiac remodeling, and signs of HF over a period of several weeks (e.g., 3-6 weeks, depending on the desired severity and endpoints).
Strengths and Limitations
Strengths:
- Clinically Relevant: Faithfully mimics human volume-overload hypertension and low-renin hypertension, a common subset of clinical hypertension.
- Robust Phenotypes: Consistently induces pronounced cardiac hypertrophy, fibrosis, and dysfunction (both diastolic and eventually systolic), providing clear measurable endpoints.
- Mechanistic Insights: Offers a clear mechanistic pathway focusing on mineralocorticoid receptor (MR) activation, inflammation, and oxidative stress as key drivers of pathology.
- Translational Value: Suitable for evaluating a broad spectrum of therapeutic agents, including MR antagonists, anti-fibrotics, anti-inflammatories, and agents targeting oxidative stress.
- HFpEF Modeling: Provides a valuable tool for studying the transition from hypertension to HF with preserved ejection fraction (HFpEF).
Limitations:
- Acute Induction: The model can induce rapid, severe hypertension, which may not fully represent the gradual progression of human essential hypertension.
- Systemic Effects: DOCA has systemic effects beyond the cardiovascular system, which might complicate the interpretation of highly specific interventions.
- Renin Suppression: Characterized by low circulating renin, which limits its applicability for studying high-renin forms of hypertension.
- Surgical Intervention: Requires surgical uninephrectomy, adding a layer of complexity compared to purely pharmacological models.
Evaluation Platform
Our comprehensive evaluation platform integrates advanced methodologies to provide a holistic understanding of disease progression and therapeutic efficacy in the DOCA & Salt model. We offer a robust suite of biochemical, molecular, cellular, histopathological, behavioral, and imaging analyses.
Key Test Indicators:
- Cardiac Function (Echocardiography & Hemodynamics): Left ventricular ejection fraction (LVEF), fractional shortening (FS), wall thickness, chamber dimensions, E/A ratio, left ventricular end-diastolic pressure (LVEDP),± dP/dt.
- Blood Pressure: Systolic, diastolic, and mean arterial pressure (telemetry or tail-cuff).
- Renal Function: Blood urea nitrogen (BUN), creatinine, urine protein, albuminuria, electrolyte levels.
- Cardiac Remodeling: Myocardial hypertrophy (heart weight/body weight ratio, cardiomyocyte cross-sectional area), collagen deposition (Masson's Trichrome, Picrosirius Red staining, collagen content), fibrosis markers (procollagen types, TGF-β1).
- Inflammation & Oxidative Stress: Levels of inflammatory cytokines (TNF-α, IL-6), oxidative stress markers (MDA, ROS), NADPH oxidase activity.
- Neurohormonal Activation: Plasma ANP, BNP, aldosterone, renin activity.
- Electrophysiology: ECG parameters (PR interval, QRS duration, QT interval).
Applications
- Simulated Diseases: Primarily models volume-overload induced hypertension, hypertensive heart disease, HFpEF, and cardiorenal syndrome. It also helps in understanding myocardial fibrosis, inflammation, and oxidative stress-related cardiovascular pathologies.
- Drug Evaluation: Ideal for assessing novel antihypertensive agents, mineralocorticoid receptor antagonists (MRAs), anti-fibrotic compounds, anti-inflammatory drugs, and anti-oxidants. It supports the evaluation of therapies aimed at improving cardiac function, reducing remodeling, and protecting renal health.
- Treatment Modalities: Applicable for testing small molecules, biologics, gene therapies, and even dietary or lifestyle interventions aimed at mitigating the progression of hypertension-induced HF. It also allows for mechanistic studies into the role of specific signaling pathways, such as the endothelin system.
Related Heart Failure Models
PA Constriction induced Right HF Model
Ascending Aortic Arch Constriction induced Post-Pressure Overload Heart Failure Model
Abdominal Aortic Stenosis induced Left HF Model
Adriamycin induced Left HF Model
Our Advantages
- Integrated Expertise: Seamlessly combine surgical model generation with comprehensive phenotyping and data analysis.
- Customized Solutions: Flexible study designs tailored precisely to your research questions and therapeutic targets.
- Translational Focus: Emphasis on clinically relevant endpoints and biomarkers to bridge preclinical findings to human trials.
- Quality and Reproducibility: Rigorous standardization ensures consistent, high-quality, and reliable data for your drug development.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs provides end-to-end preclinical research services utilizing the DOCA & Salt-induced left HF model. If you seek robust, translatable data to advance your therapeutic compounds, we invite you to contact us. Our team is ready to discuss your specific project needs and explore how our expertise can accelerate your research goals.
FAQs
-
Q1: What is the primary mechanism by which the DOCA & Salt model induces HF?
A: The model primarily induces HF through sustained mineralocorticoid receptor (MR) activation by DOCA, combined with high dietary salt intake and unilateral nephrectomy. This leads to volume overload, hypertension, and a cascade of pathological events including cardiac hypertrophy, inflammation, oxidative stress, and fibrosis. These processes culminate in impaired cardiac function, initially diastolic and progressing to systolic dysfunction.
-
Q2: Can this model be used to study both HFpEF and HFrEF?
A: Yes, the DOCA & Salt model is particularly valuable for studying both forms of HF. Early in its progression, it often exhibits features consistent with HFpEF, such as concentric hypertrophy and diastolic dysfunction. As the disease advances, some animals may transition to HF with reduced ejection fraction (HFrEF) with systolic impairment, allowing for investigations into the full spectrum of the disease.
-
Q3: What are the critical control groups typically included in a DOCA & Salt study?
A: Essential control groups typically include sham-operated animals (undergoing nephrectomy but no DOCA/salt treatment) to account for surgical effects, and DOCA-treated animals without salt overload (or vice versa) to delineate the synergistic effects of DOCA and salt. Vehicle controls are also crucial for testing specific therapeutic interventions.
-
Q4: Is the DOCA & Salt model suitable for screening novel anti-fibrotic compounds?
A: Absolutely. The DOCA & Salt model induces significant myocardial and renal fibrosis, making it an excellent platform for screening and evaluating novel anti-fibrotic compounds. Researchers can assess the reduction in collagen deposition, modulation of pro-fibrotic pathways (e.g., TGF-β1), and improvement in cardiac compliance.
-
Q5: Are there specific challenges associated with the long-term maintenance of DOCA & Salt animals?
A: Long-term maintenance can present challenges, including potential for severe hypertension-related complications, fluid-electrolyte imbalances, and increased mortality at advanced stages. Careful monitoring of animal welfare, fluid intake, and body weight is essential, and study durations are often optimized to balance disease progression with animal well-being.
-
Q6: Can the DOCA & Salt model provide insights into cardiorenal syndrome?
A: Yes, the DOCA & Salt model is highly valuable for studying cardiorenal syndrome. The combination of mineralocorticoid excess and hypertension induces both cardiac and renal damage, leading to a vicious cycle where dysfunction in one organ exacerbates the other. This allows for investigation of therapeutic strategies that target both cardiac and renal components of the syndrome.
Published Data
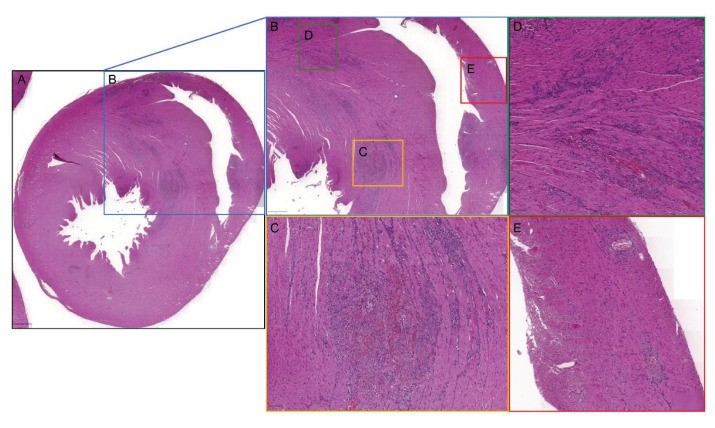 Fig.1 Histological changes in remodelled myocardium within the DOCA/salt rat model.1
Fig.1 Histological changes in remodelled myocardium within the DOCA/salt rat model.1
In a representative study utilizing the DOCA & Salt model, researchers investigated myocardial remodeling and its impact on electrophysiological properties in rats. Animals subjected to unilateral nephrectomy, DOCA administration, and increased salt intake demonstrated significant structural changes, including increased left ventricular wall thickness and myocardial fibrosis. Notably, the study revealed distinct electrophysiological alterations, such as prolongation of the PR interval and widening of the QRS complex, indicating disrupted cardiac conduction. These findings underscore the model's utility in dissecting the intricate interplay between structural remodeling and electrical dysfunction in HF.
Reference
- Laska, M et al. "Heart remodelling affects ECG in rat DOCA/salt model." Physiological research vol. 73,S3 (2024): S727-S753. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.33549/physiolres.935512
For Research Use Only.
