Foreign Matter induced Arterial Thrombosis Modeling & Pharmacodynamics Service
Introduction
Thrombosis, the formation of an unwanted blood clot within a blood vessel, is a critical pathological process underlying conditions such as myocardial infarction and ischemic stroke. These arterial events are primarily driven by platelet-rich thrombi forming at sites of vascular injury. Effective antithrombotic therapies are essential for preventing and treating these life-threatening diseases.
At Creative Biolabs, we provide a diverse array of well-established preclinical models designed to rigorously evaluate the efficacy of novel antithrombotic compounds.
Foreign Matter-Induced Arterial Thrombosis Model
The foreign matter-induced arterial thrombosis model is a highly versatile and clinically relevant in vivo platform used to study arterial thrombus formation initiated by the presence of foreign materials or chemical irritants. This model accurately recapitulates thrombotic events seen in clinical settings, such as those associated with medical devices or vascular injury. It serves as an indispensable tool for assessing the antithrombotic potential of new drug candidates, evaluating the biocompatibility of novel biomaterials, and optimizing the properties of drug-eluting coatings designed to prevent device-related thrombosis.
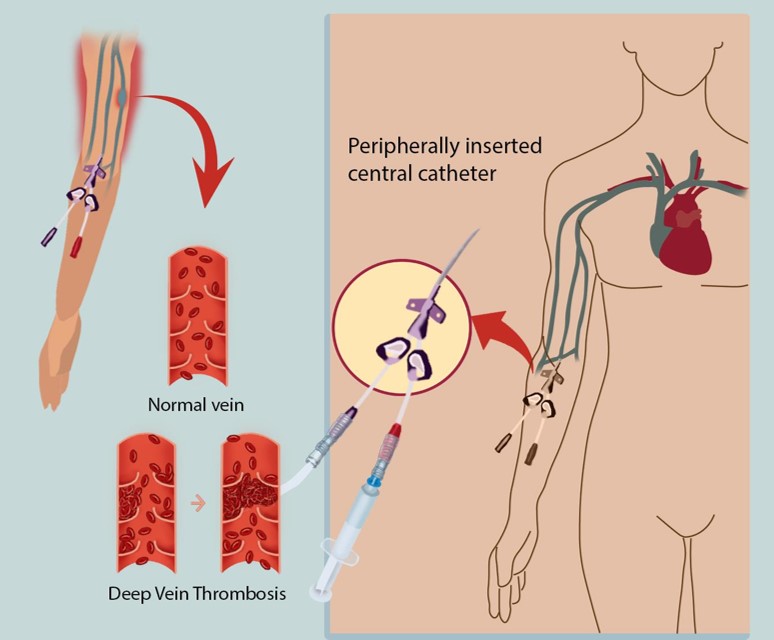 Fig.1 Illustration of catheter-induced thrombosis.1
Fig.1 Illustration of catheter-induced thrombosis.1
Model Construction Steps
The construction strategy for foreign matter-induced arterial thrombosis models generally involves the direct implantation of a thrombogenic foreign body (e.g., a stent, a coated wire, or a catheter) into a target artery, often in rodents. The foreign material itself provides the surface and stimulus for thrombus formation, ensuring a reproducible and quantifiable thrombotic response.
01Anesthesia and Surgical Exposure
The rodent (commonly mouse or rat) is anesthetized, and a specific artery (e.g., carotid, femoral) is surgically exposed.
02Foreign Material Implantation
A foreign body, such as a stent, a coated wire, or a catheter, is carefully implanted directly into the arterial lumen. This material serves as the primary thrombogenic stimulus, initiating thrombus formation on its surface.
03Thrombus Monitoring
Thrombus formation and growth are monitored in real-time, often using doppler flowmetry to measure blood flow reduction or cessation, or by direct visualization.
04Endpoint Collection
After a defined observation period, the vessel is harvested for ex vivo analysis, or the animal is observed for long-term outcomes.
Strengths and Limitations
Strengths:
- High Clinical Relevance: Directly mimics human arterial thrombotic conditions, especially those related to medical devices.
- Reproducible Thrombus Formation: Provides consistent and quantifiable results, enabling robust statistical analysis.
- Quantitative Readouts: Allows for precise measurement of thrombus size, time to occlusion, and other critical parameters.
Limitations:
- Invasive Procedure: Requires surgical expertise and careful animal handling.
- Acute Focus: Primarily models acute thrombotic events, though chronic studies are possible with modifications.
- Cost and Throughput: Generally more resource-intensive than in vitro assays.
Evaluation Platform
Creative Biolabs' state-of-the-art evaluation platform integrates advanced biochemical, molecular, cellular, histopathological, and imaging techniques to provide a comprehensive assessment of thrombotic processes. Our laboratories are equipped with cutting-edge instruments to ensure precise and reliable data acquisition, enabling a holistic understanding of drug effects and material interactions within the complex vascular environment.
Key Test Indicators:
- Time to Occlusion (TTO): Measured by doppler flowmetry.
- Thrombus Weight/Size: Gravimetric or imaging-based quantification.
- Vessel Patency: Assessment of sustained blood flow.
- Histopathological Analysis: Thrombus morphology, composition (platelets, fibrin, red blood cells), and vessel wall damage.
- Immunohistochemistry: Localization and quantification of specific proteins (e.g., platelet markers, fibrin, inflammatory cells).
- Ex Vivo Platelet Aggregation: Assessment of drug effects on platelet function.
- Coagulation Assays: Prothrombin Time (PT), Activated Partial Thromboplastin Time (aPTT), Thrombin Generation Assay.
- Flow Cytometry: Analysis of circulating platelet activation markers.
Applications
- Simulating Diseases: This model accurately simulates critical cardiovascular diseases, including stent thrombosis, other medical device-related thrombotic events (e.g., from catheters or prosthetic valves), and acute arterial occlusion. It offers a realistic in vivo environment to study disease progression and therapeutic interventions.
- Evaluating Drugs: The model is essential for rigorously evaluating novel antithrombotic drugs, such as antiplatelet agents, anticoagulants, fibrinolytic therapies, and combination treatments. It provides quantitative readouts of thrombus formation and dissolution, aiding in determining optimal dosing and mechanisms.
- Assessing Treatments: Crucially, the model assesses new biomaterials' biocompatibility and optimizes drug-eluting coatings on medical devices. It also investigates the thrombotic implications of new surgical techniques, ensuring advancements minimize adverse thrombotic events.
Related Thrombosis Models
- Transient Blood Flow Occlusion induced Inferior Vena Cava Thrombosis Model
- Thrombin induced Inferior Vena Cava Thrombosis Model
- Arteriovenous Fistula Thrombosis Model
- Fe2O3 induced Arterial Thrombosis Model
- Ferric Chloride induced Thrombosis Model
Our Advantages
- Customizable Protocols: Tailored study designs to meet unique client objectives and compound characteristics.
- Comprehensive Analytical Capabilities: Extensive range of readouts from real-time monitoring to detailed histopathology.
- Translational Focus: Data generated is highly predictive of clinical outcomes, accelerating your drug development pipeline.
- Dedicated Scientific Team: Collaboration with expert biologists providing insightful data interpretation and strategic guidance.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is dedicated to advancing your antithrombotic research with our unparalleled expertise and comprehensive service offerings. Our Foreign Matter-Induced Arterial Thrombosis Model provides robust, clinically relevant data to accelerate your drug candidates' journey. We invite you to contact us for a personalized consultation to discuss how our capabilities can support your specific project needs.
FAQs
-
Q1: Can this model be used to evaluate the long-term patency of a vessel after a thrombotic event?
A: While primarily for acute thrombosis, this model can assess long-term outcomes. Extending the observation period to weeks or months allows evaluation of re-endothelialization, vessel remodeling, and sustained patency, providing insights into chronic effects and re-thrombosis. Careful consideration of foreign material and injury severity ensures suitability for chronic evaluation.
-
Q2: What animal species are typically used for the Foreign Matter-Induced Arterial Thrombosis Model, and why?
A: Mice and rats are commonly used due to their genetic tractability, lower cost, and ease of handling. While their coagulation systems differ from humans, they offer a reproducible platform for initial efficacy and mechanism of action studies. Larger animals like rabbits or pigs may be chosen for later-stage studies requiring larger vessels or complex device implantation.
-
Q3: Is it possible to combine this model with other disease models, such as atherosclerosis?
A: Yes, integrating this model with pre-existing conditions like atherosclerosis enhances clinical relevance. Inducing thrombosis in atherosclerotic vessels better mimics human plaque rupture events, allowing evaluation of antithrombotic agents in a more complex, clinically representative environment where underlying vascular pathology contributes to thrombotic risk.
-
Q4: What types of readouts are most critical for assessing the efficacy of an antiplatelet drug in this model?
A: For antiplatelet drug efficacy, critical readouts include time to stable occlusion, directly reflecting the drug's ability to prevent platelet plug formation. Ex vivo platelet aggregation assays confirm direct impact on platelet function. Histopathological analysis of thrombus composition, specifically platelet content, further validates the antiplatelet effect within the vessel.
-
Q5: Can this model differentiate between systemic and local antithrombotic effects?
A: Yes, the model can differentiate these effects. Systemic efficacy is assessed by administering a drug systemically and observing its impact on a localized thrombus. For local effects, the foreign material itself can be drug-coated, allowing direct evaluation of localized antithrombotic properties without significant systemic exposure. This distinction is vital for optimizing drug delivery.
-
Q6: Beyond drug efficacy, how can this model assist in medical device development?
A: For medical device development, this model is invaluable for assessing new materials' thrombogenicity and surface modifications. By implanting prototype devices or material samples, we directly evaluate their interaction with blood components, quantifying thrombus burden. This enables iterative design improvements, ensuring new devices are functional, highly biocompatible, and minimize device-related thrombosis.
Published Data
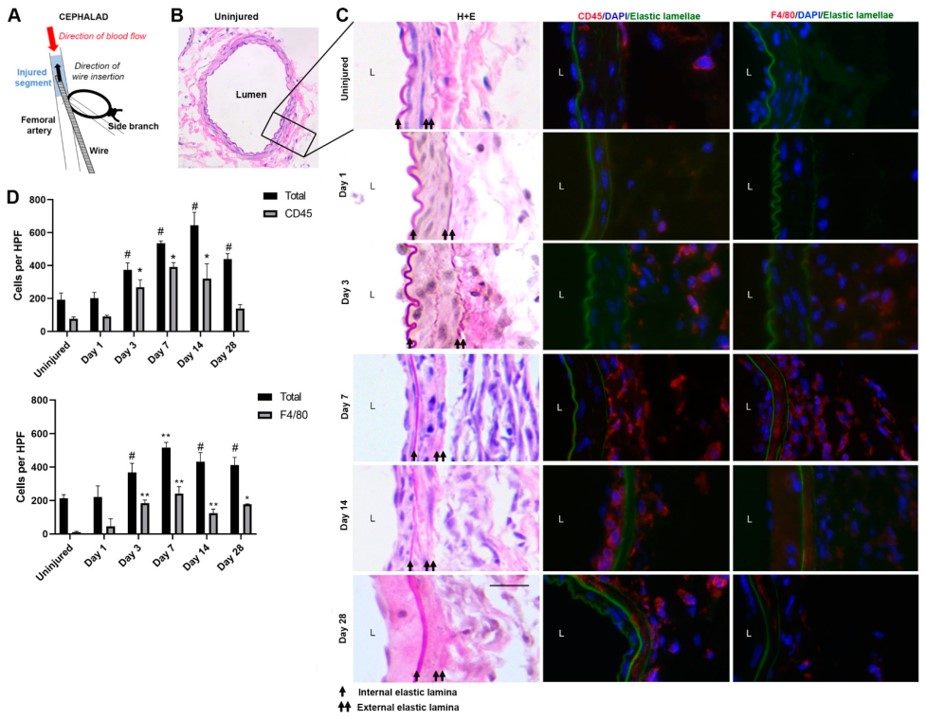 Fig.2 Establishment of femoral artery wire injury.2
Fig.2 Establishment of femoral artery wire injury.2
The study utilized a wire injury model of the femoral artery to investigate inflammatory cell dynamics after injury and their role in neointimal hyperplasia. The research provided detailed insights into the cellular responses post-injury, demonstrating the model's utility in understanding vascular remodeling and potential therapeutic targets beyond acute thrombosis.
References
- Cardoso, Patrícia Cristina et al. "Biomarkers Associated with Thrombosis in Patients with Peripherally Inserted Central Catheter: A Systematic Review and Meta-Analysis." Journal of clinical medicine vol. 12,13 4480. 4 Jul. 2023. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image. https://doi.org/10.3390/jcm12134480
- Pamulapati, Vivek et al. "Inflammatory Cell Dynamics after Murine Femoral Artery Wire Injury: A Multi-Parameter Flow Cytometry-Based Analysis." Cells vol. 12,5 689. 22 Feb. 2023. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/cells12050689
For Research Use Only.
