Indomethacin induced Small Intestinal Inflammatory Modeling & Pharmacodynamics Service
Introduction
Colitis refers to inflammation of the colon and is a key feature of inflammatory bowel disease (IBD), a chronic relapsing condition that includes ulcerative colitis (UC) and Crohn's disease (CD). While UC is typically confined to the colon and rectum, Crohn's disease can affect any part of the gastrointestinal tract, often presenting with transmural inflammation. Both diseases involve a complex interplay of genetic, environmental, microbial, and immune factors, leading to symptoms such as abdominal pain, diarrhea, weight loss, and rectal bleeding. The pathological hallmarks of colitis include epithelial injury, immune cell infiltration, crypt distortion, and mucosal ulceration. Due to the heterogeneity of colitis in clinical presentation and etiology, various rodent models have been developed to simulate different aspects of the disease, including chemically induced, genetically modified, and adoptive transfer models. These models are invaluable tools for understanding disease mechanisms and for preclinical evaluation of anti-inflammatory, immunosuppressive, and microbiota-targeted therapies. Creative Biolabs offers a wide range of well-established and customizable rodent colitis models, providing comprehensive platforms for evaluating the efficacy and mechanisms of potential therapeutic agents under physiologically relevant conditions.
Disease Models and Applications
The Indomethacin-induced rodent small intestinal inflammatory model is established by administering indomethacin to rodents, which induces an inflammatory response in the small intestine. This model replicates the pathological changes such as mucosal damage, edema, and inflammatory cell infiltration commonly observed in NSAID-induced gastrointestinal injury. Indomethacin induces oxidative stress and disrupts the integrity of the intestinal epithelium, triggering a cascade of inflammatory cytokines. The model is valuable for studying the mechanisms of NSAID-induced intestinal injury, testing the efficacy of anti-inflammatory drugs, and screening for potential protective agents like COX inhibitors or mucosal protectants. Its primary strength lies in its reproducibility and ability to mimic the clinical effects of NSAIDs on the small intestine. However, a limitation is that it may not fully replicate the complex multifactorial nature of gastrointestinal inflammation seen in human diseases.
- Simulates: This model mimics the pathological features of NSAID-induced small intestinal inflammation, including mucosal damage, inflammatory cell infiltration, and oxidative stress.
- Evaluates Drugs: This model is used to evaluate drugs aimed at treating NSAID-induced small intestinal inflammation, including COX inhibitors, anti-inflammatory agents, mucosal protectants, and antioxidant therapies.
Measurements
We offer a comprehensive range of measurements for evaluating drug efficacy in the indomethacin-induced rodent small intestinal inflammatory model, utilizing advanced techniques, including but not limited to:
- General observations: Body weight, intestinal inflammation score, food/water intake, mortality rate, and signs of gastrointestinal distress.
- Histopathological analysis: Assessment of intestinal tissue damage, including mucosal erosion, inflammation, ulceration, and hemorrhage through hematoxylin and eosin (H&E) staining.
- Endoscopic evaluation: Visualization and measurement of mucosal damage and inflammatory lesions in the small intestine.
- Cytokine profiling (e.g., ELISA): Measurement of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which are elevated in inflammatory responses.
- Oxidative stress markers: Measurement of reactive oxygen species (ROS) and antioxidant enzymes like superoxide dismutase (SOD), catalase, and glutathione peroxidase.
- Gene/protein expression profiling via RT qPCR and Western blot techniques: Analysis of COX-2, NF-κB, and other inflammatory markers to assess the degree of tissue inflammation and repair.
In addition to the established model, we also offer the development of customized rodent models based on previous literature and studies. Our scientific team is available for guidance in experimental design, model selection, and data analysis, ensuring a tailored approach to your specific research needs.
Related Services
In addition to the indomethacin-induced rodent small intestinal inflammatory model, we also offer a range of other colitis models for investigating different mechanisms of intestinal inflammation, including:
- TNBS/DNBS induced Colitis Model
- DSS induced Colitis Model
- OXA induced Colitis Model
- Acetic Acid induced IBD Model
- Anti-CD40 Ab induced IBD Model
- IL-10 KO Mouse Spontaneous IBD Model
- CD4+CD45RBhi T Cells induced IBD Model
Advantages
- Expertise and Experience: We bring years of specialized knowledge in preclinical models and offer high-quality, reliable, and scientifically sound models for NSAID-induced gastrointestinal research.
- Comprehensive Services: From experimental design to data analysis and interpretation, we offer full support throughout your project to ensure a tailored approach.
- Cutting-Edge Technologies: Our advanced technologies, including cytokine profiling, histopathological analysis, and gene/protein expression profiling, ensure the production of meaningful and accurate results.
- Customized Models: In addition to established models, we offer the flexibility to develop novel, customized models based on your specific research requirements.
- Collaborative Approach: Our scientific team works closely with you to ensure the study design and methodology align with your research goals.
- Reliable Results: Our rigorous quality control processes and standardized procedures ensure consistent and reproducible results, enabling reliable data for drug evaluation.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: How long does it take to conduct a study using your indomethacin-induced small intestinal inflammation models?
A1: The duration of the study depends on the specific experimental design and the scope of your project. Typically, studies can range from a few weeks to a couple of months, including data collection and analysis.
-
Q2: What support do you provide during the study?
A2: Our scientific team provides full support, including experimental design, model selection, data analysis, and interpretation, ensuring a smooth and efficient research process.
-
Q3: Do you offer other disease models beyond small intestinal inflammation?
A3: Yes, we offer a variety of rodent disease models, including those for liver disease, cancer, atherosclerosis, and kidney fibrosis, among others.
-
Q4: How do I get started with your services?
A4: Simply contact us to discuss your research objectives. Our team will guide you through model selection, study design, and timeline to kickstart your project.
-
Q5: Are your models validated for publication?
A5: Yes, all our models are validated based on well-established scientific protocols and are suitable for generating publishable results.
Published Data
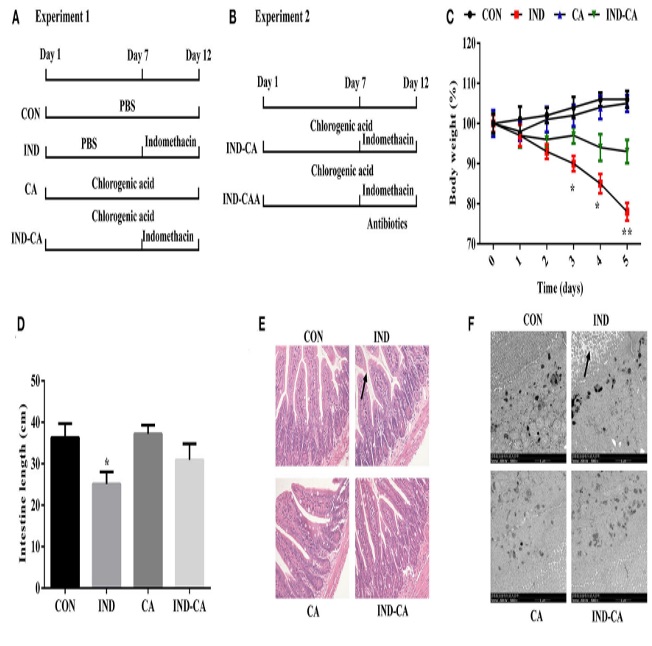 Fig. 1 Chlorogenic acid prevented morphological damage to intestines of indomethacin-treated mice.1
Fig. 1 Chlorogenic acid prevented morphological damage to intestines of indomethacin-treated mice.1
This experimental project aimed to explore the influence of chlorogenic acid (CGA) on gut microbiota and their metabolites, as well as its potential therapeutic effects and mechanisms in inflammatory bowel disease. Indomethacin was used to induce intestinal inflammation in mice, followed by oral administration of CGA. To further investigate the role of intestinal microbiota, fecal microbiota transplantation was performed after treatment. The experimental design was outlined in two separate protocols (A: Experiment 1; B: Experiment 2). Key outcome measures included body weight changes post-indomethacin administration (C), intestinal length (D), colon morphology assessed by hematoxylin and eosin (H&E) staining (E), and ultrastructural analysis of microvilli in the colon using transmission electron microscopy (TEM) (F). Morphological alterations were highlighted with black arrows in images (E) and (F). Results indicated that CGA effectively prevented indomethacin-induced structural damage in the intestines, suggesting its protective role potentially through modulation of gut microbial composition and function.
Reference
- Yan, Yongwang et al. "Chlorogenic Acid Protects Against Indomethacin-Induced Inflammation and Mucosa Damage by Decreasing Bacteroides-Derived LPS." Frontiers in Immunology vol. 11 1125. 3 Jun. 2020, doi:10.3389/fimmu.2020.01125. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
