Sodium Taurocholate induced Acute Pancreatitis Modeling & Pharmacodynamics Service
Introduction
Acute pancreatitis (AP) is an acute inflammatory disorder of the pancreas characterized by sudden onset of abdominal pain, elevated serum pancreatic enzymes (amylase and lipase), and varying degrees of pancreatic tissue injury. It is a common gastrointestinal emergency and may progress from a mild, self-limiting condition to severe necrotizing pancreatitis associated with systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), and high mortality. The two main clinical subtypes are interstitial edematous pancreatitis, accounting for most mild cases, and necrotizing pancreatitis, which involves pancreatic necrosis and severe systemic complications. The pathogenesis of AP involves premature activation of digestive enzymes within pancreatic acinar cells, leading to autodigestion, inflammation, and recruitment of immune cells. This cascade results in local tissue damage and release of inflammatory mediators such as TNF-α, IL-1β, and IL-6, which can trigger systemic effects and distant organ injury. Common etiological factors include gallstones, excessive alcohol consumption, hypertriglyceridemia, certain medications, and infections. Currently, there is no specific pharmacological treatment for AP; management mainly involves supportive care, fluid resuscitation, and treating the underlying cause. Therefore, there is an urgent need for effective therapeutic strategies that can control inflammation, prevent necrosis, and protect organ function. Creative Biolabs provides multiple well-characterized rodent models of acute pancreatitis. Our services include model induction, clinical and histological evaluation, cytokine analysis, enzyme assays, and systemic toxicity assessments. With advanced analytical tools and experienced scientists, we offer reliable and customized solutions to support your preclinical drug development targeting acute pancreatitis.
Disease Models and Applications
The Sodium Taurocholate-Induced Acute Pancreatitis (AP) Model is a widely used rodent model that mimics the pathophysiology of human acute pancreatitis, particularly biliary-induced pancreatitis. This model is established by retrogradely injecting sodium taurocholate, a bile acid, into the biliopancreatic duct of rodents, leading to pancreatic injury, inflammation, and edema. The administration of sodium taurocholate results in rapid activation of pancreatic enzymes, acinar cell injury, and recruitment of inflammatory cells, mimicking key features of human AP. The model is advantageous because it induces both local pancreatic inflammation and systemic inflammatory responses, including cytokine release and oxidative stress, making it suitable for evaluating therapeutic interventions targeting multiple aspects of the disease. Additionally, it can replicate severe forms of pancreatitis with complications like acinar necrosis and organ dysfunction. However, its drawbacks include the technical complexity of the procedure, as it requires precise injection into the biliopancreatic duct. Furthermore, while it effectively models biliary-induced pancreatitis, it may not fully replicate other etiologies of AP, such as alcohol-induced pancreatitis. Despite these limitations, the Sodium Taurocholate-Induced AP Model remains a valuable tool for preclinical studies focused on drug development for acute pancreatitis.
Simulates: The Sodium Taurocholate-Induced Acute Pancreatitis Model simulates severe acute pancreatitis, particularly biliary-induced forms caused by gallstone obstruction. It replicates key pathological features such as acinar cell necrosis, pancreatic inflammation, systemic cytokine release, and multi-organ involvement, closely resembling human necrotizing pancreatitis.
Evaluates Drugs: This model is used to evaluate a variety of therapeutic candidates, including anti-inflammatory agents, cytokine inhibitors (e.g., anti-TNF-α, IL-1β blockers), antioxidant compounds, protease inhibitors, and organ-protective drugs. It is particularly suitable for testing treatments aimed at reducing pancreatic injury, systemic inflammation, and associated complications in acute pancreatitis.
Measurements
We offer a comprehensive array of measurements for evaluating drug efficacy in the sodium taurocholate-induced acute pancreatitis model, utilizing advanced technologies and evidence-based protocols, including but not limited to:
- General observations: Body weight loss, abdominal distension, reduced activity, and survival rate monitoring.
- Serum enzyme assays: Quantification of pancreatic enzymes such as amylase and lipase to assess the extent of pancreatic injury.
- Histopathological evaluation: Hematoxylin and eosin (H&E) staining of pancreatic tissue to examine edema, leukocyte infiltration, acinar cell necrosis, and hemorrhage.
- Cytokine profiling (e.g., ELISA): Measurement of systemic and local inflammatory mediators, including TNF-α, IL-1β, IL-6, and MCP-1.
-
Myeloperoxidase (MPO) activity assay: To evaluate neutrophil infiltration in the pancreas and distant organs.
• Oxidative stress biomarkers: Assessment of malondialdehyde (MDA) levels, glutathione (GSH), and superoxide dismutase (SOD) activity in pancreatic tissue. - Organ function assessment: Measurement of liver (ALT, AST) and kidney (creatinine, BUN) function markers to detect systemic effects.
- Gene/protein expression profiling: RT-qPCR and Western blot analysis of inflammatory signaling molecules, apoptotic markers, and protective proteins in pancreatic and peripheral tissues.
Our team provides end-to-end support in experimental planning, model optimization, sample collection, and data interpretation. With robust protocols and advanced analytics, we deliver high-quality preclinical data to accelerate your acute pancreatitis drug development.
Related Services
In addition to the sodium taurocholate-induced acute pancreatitis model, we offer a variety of other acute pancreatitis models, each designed to meet specific research needs. Our team provides expert support to ensure tailored, high-quality results.
- Cerulein induced Acute Pancreatitis Model
- Caerulein & LPS induced Acute Pancreatitis Model
Advantages
- Comprehensive Model Portfolio: We offer a diverse range of validated rodent models, including those for acute pancreatitis, IBD, and other gastrointestinal diseases, tailored to your specific research objectives.
- Customizable Solutions: Our scientific team works closely with you to customize experimental designs, dosing regimens, and evaluation parameters to match your drug development needs.
- Advanced Analytical Technologies: We utilize cutting-edge tools like ELISA, RT-qPCR, Western blot, flow cytometry, and histopathology to provide reliable, detailed insights into drug efficacy and mechanisms.
- Experienced Expertise: With years of experience in preclinical research, our experts provide guidance at every stage, from study design to data analysis, ensuring optimal results and timely delivery.
- High-Quality and Reproducible Data: We follow standardized protocols and rigorous quality control measures, ensuring that every study produces reliable, reproducible results to support your regulatory filings and research goals.
- Efficient Project Execution: We offer fast turnaround times while maintaining high scientific standards, delivering actionable results quickly to accelerate your research progress.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What types of acute pancreatitis models do you provide?
A1: We offer several well-established rodent models of acute pancreatitis, including sodium taurocholate-induced, cerulein-induced, and L-arginine-induced models. Each is suited for studying different disease mechanisms and severities.
-
Q2: Can you help select the most suitable model for our study?
A2: Yes. Based on your therapeutic target and research objectives, our scientific team will recommend the most appropriate model and evaluation strategy to maximize the relevance and reliability of your data.
-
Q3: What parameters can you evaluate in pancreatitis studies?
A3: We assess a wide range of indicators, including serum amylase/lipase levels, pancreatic histopathology, inflammatory cytokine levels (e.g., TNF-α, IL-6), myeloperoxidase activity, oxidative stress markers, and organ function biomarkers (e.g., ALT, AST, BUN, creatinine).
-
Q4: Do you offer customized study designs?
A4: Absolutely. We tailor the study protocol, dosing schedules, administration routes, and assessment time points to align with your specific therapeutic mechanism and research timeline.
-
Q5: How do you ensure data quality and reproducibility?
A5: We follow validated protocols with strict quality control procedures, including proper controls, standardized scoring systems, and trained technical staff to ensure consistency and high data integrity.
Published Data
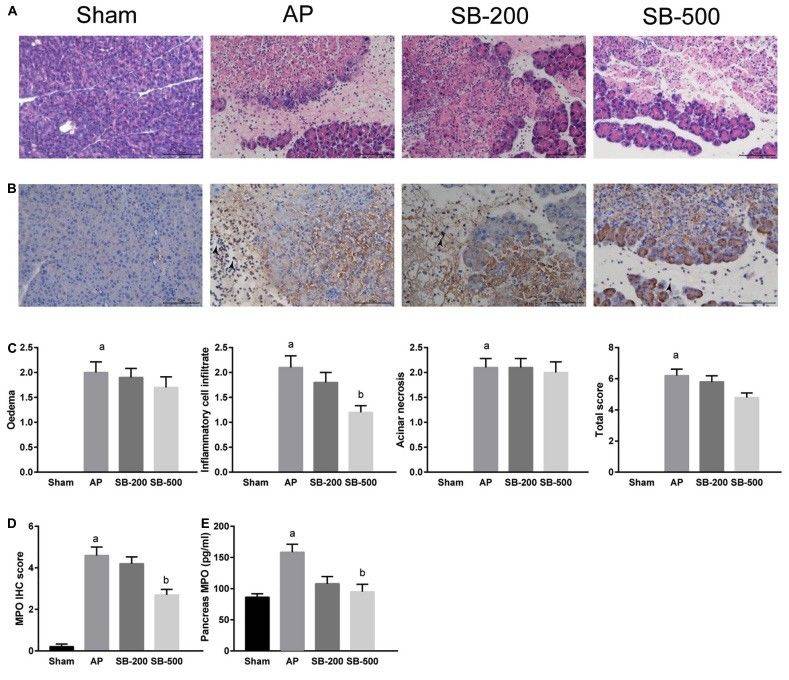 Fig.1 HE and IHC staining, and concentration of MPO in the pancreatic tissue of mice in each group.1
Fig.1 HE and IHC staining, and concentration of MPO in the pancreatic tissue of mice in each group.1
This study investigated the effects of sodium butyrate (SB) on inflammation in pancreatic tissue using a mouse model of acute pancreatitis (AP). Histological analysis revealed that mice in the AP group exhibited severe pathological changes, including loss of normal acinar architecture, diffuse acinar cell necrosis, pronounced interlobular septal edema, and extensive inflammatory cell infiltration with fibrin exudation (Figure 1A). Treatment with 500 mg/kg sodium butyrate (SB500) markedly reduced inflammatory cell infiltration and fibrin deposition, and slightly alleviated interlobular edema, although pancreatic necrosis remained largely unchanged compared to the AP group (Figures 1A,1C). In contrast, the 200 mg/kg sodium butyrate group (SB200) showed no significant improvement in either inflammatory infiltration or necrosis (Figures 1A, 1C). Immunohistochemical staining for myeloperoxidase (MPO) demonstrated a significant reduction in MPO-positive cells in the SB500 group relative to the AP group (Figures 1B, 1D), while the SB200 group showed no statistical difference. ELISA quantification of MPO levels in pancreatic tissue confirmed these findings (Figure 1E). These results indicate that high-dose sodium butyrate alleviates pancreatic inflammation primarily by reducing neutrophil infiltration.
Reference
- Xiong, Yangyang et al. "Sodium Butyrate Attenuates Taurocholate-Induced Acute Pancreatitis by Maintaining Colonic Barrier and Regulating Gut Microorganisms in Mice." Frontiers in Physiology vol. 13 813735. 17 Mar. 2022, DOI:10.3389/fphys.2022.813735. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
