Transient Blood Flow Occlusion induced Inferior Vena Cava Thrombosis Modeling & Pharmacodynamics Service
Introduction
Thrombosis, the formation of pathological blood clots, represents a significant global health challenge, leading to conditions like deep vein thrombosis (DVT) and pulmonary embolism (PE). These events are influenced by complex interactions of blood flow, vessel wall integrity, and coagulation factors. Accurate preclinical models are essential for understanding disease mechanisms and developing effective therapies.
Creative Biolabs is proud to offer a comprehensive suite of well-established and highly predictive in vivo thrombosis models to evaluate the efficacy of novel antithrombotic and thrombolytic agents.
Transient Blood Flow Occlusion-Induced IVC Thrombosis Modelig.2 Validation of mouse DVT models induc
The transient blood flow occlusion-induced inferior vena cava (IVC) thrombosis Model is designed to mimic the multifactorial pathogenesis of human venous thromboembolism (VTE). This model induces a controlled, temporary cessation of blood flow in the IVC, followed by reperfusion. This process creates a mild endothelial perturbation and a period of localized stasis, fostering the formation of a stable, fibrin-rich thrombus that closely resembles clinical venous clots. It serves as an invaluable platform for investigating the initiation, propagation, and resolution of thrombi, as well as for evaluating the efficacy of potential therapeutic interventions.
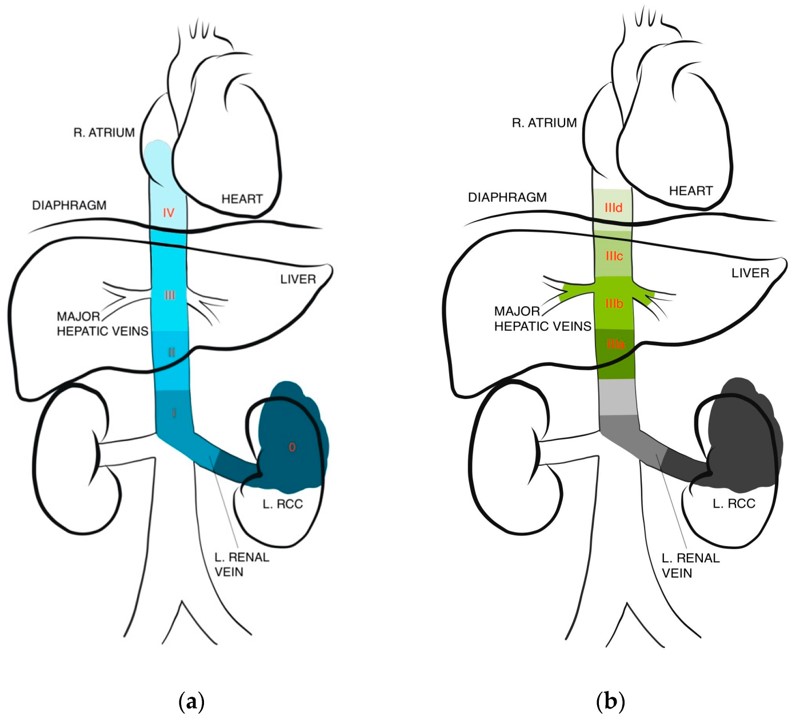 Fig.1 IVC thrombus level classifications.1
Fig.1 IVC thrombus level classifications.1
Model Construction Steps
The construction of this model involves a precise surgical approach to induce transient blood flow occlusion. The general strategy focuses on temporarily impeding blood flow in the IVC to trigger a thrombotic response without causing severe, unphysiological vessel damage.
01Anesthesia and Surgical Exposure
Rodents (typically mice or rats) are anesthetized, and a midline laparotomy is performed to expose the IVC and surrounding tissues, usually just distal to the renal veins.
02Branch Cauterization
Any collateral branches draining into the IVC in the target area are carefully identified and cauterized to ensure consistent blood flow dynamics and prevent thrombus termination at these sites.
03Transient Occlusion Device Placement
A non-traumatic surgical clip or a fine ligature is carefully positioned around the IVC.
04Controlled Occlusion
The IVC is completely occluded for a defined, short period (e.g., 5-15 minutes). This duration is critical for inducing sufficient stasis and mild endothelial activation.
05Reperfusion
Following the timed occlusion, the clip or ligature is gently removed, allowing blood flow to resume. The combination of transient stasis and reperfusion-induced mild injury initiates thrombus formation.
06Closure and Recovery
The surgical site is closed, and the animals are allowed to recover, with thrombus development monitored over subsequent days.
Strengths and Limitations
Strengths:
- Physiological Relevance: The model closely recapitulates the conditions leading to spontaneous VTE in humans, involving a combination of altered flow and subtle endothelial activation, yielding thrombi rich in fibrin and red blood cells.
- Maintained Blood Flow: Unlike complete ligation models, this approach allows for the preservation of blood flow post-occlusion, enabling a more accurate assessment of systemically administered drugs.
- Robust Reproducibility: Standardized protocols ensure consistent thrombus formation, leading to reliable and statistically significant data.
- Versatile Parameters: The duration of occlusion and other experimental variables can be precisely controlled, allowing for tailored study designs.
- Translational Potential: Findings are more likely to translate successfully to clinical trials due to the model's closer resemblance to human pathophysiology.
Limitations:
- Surgical Skill Requirement: The precise nature of the surgical procedure demands experienced personnel to ensure consistent results and minimize variability.
- Variability in Some Sub-models: While generally reproducible, specific variations of flow restriction models (e.g., certain stenosis techniques) can exhibit thrombus size variability if not meticulously controlled.
- Absence of Pre-existing Conditions: The model typically induces de novo thrombosis and may not fully capture the complexity of DVT in patients with pre-existing conditions unless specifically integrated.
Evaluation Platform
Creative Biolabs' comprehensive evaluation platform supports the Transient Blood Flow Occlusion-Induced IVC Thrombosis Model with state-of-the-art techniques, including biochemical, molecular, cellular, and histopathological analyses, alongside advanced imaging.
Key Test Indicators:
- Thrombus Size: Weight, length, and volume measurements.
- Thrombus Composition: Fibrin, red blood cell, platelet, macrophage, and collagen content, assessed via histology and immunohistochemistry.
- Vessel Dynamics: Patency and recanalization rates.
- Inflammatory Markers: Indicators of inflammation and endothelial activation.
Applications
- Simulated Diseases: Primarily simulates DVT and aspects of VTE, including cancer-associated thrombosis (CAT).
- Drug Evaluation: Ideal for screening and evaluating novel antithrombotic agents (e.g., anticoagulants, antiplatelet drugs) and thrombolytic agents.
- Mechanism of Action Studies: Elucidating the cellular and molecular pathways involved in thrombus formation, propagation, and resolution.
- Combination Therapy Assessment: Evaluating synergistic effects of different therapeutic regimens.
- Biomarker Discovery: Identifying and validating potential biomarkers for thrombosis risk, progression, or therapeutic response.
- Intervention Efficacy: Assessing the impact of various treatments on thrombus resolution and vessel recanalization.
Related Thrombosis Models
- Thrombin induced Inferior Vena Cava Thrombosis Model
- Arteriovenous Fistula Thrombosis Model
- Fe2O3 induced Arterial Thrombosis Model
- Foreign Matter induced Arterial Thrombosis Model
- Ferric Chloride induced Thrombosis Model
Our Advantages
- Unparalleled Expertise: Decades of specialized experience in designing and executing complex in vivo thrombosis studies.
- Advanced Facilities: State-of-the-art surgical suites, imaging, and analytical platforms ensuring high-quality, reliable data.
- Customized Study Designs: Flexible and bespoke study protocols tailored precisely to your unique research objectives.
- Rigorous Data Analysis: Comprehensive data interpretation and robust reporting for publication-ready results.
- Ethical Compliance: Strict adherence to ethical guidelines and quality control for data integrity and animal welfare.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is dedicated to accelerating your thrombosis research with our advanced Transient Blood Flow Occlusion-Induced IVC Thrombosis Model Services. Our expertise and robust platforms are designed to provide the precise data you need for your next breakthrough. We invite you to connect with our scientific team to discuss your specific project requirements.
FAQs
-
Q1: How does the transient blood flow occlusion-induced IVC thrombosis model differ from traditional ligation models?
A: This model distinguishes itself by inducing a temporary, controlled occlusion followed by reperfusion, which allows for the maintenance of blood flow in the vessel after the initial thrombotic event. In contrast, traditional ligation models involve a complete and permanent cessation of blood flow, which may not accurately reflect the physiological conditions under which most human deep vein thromboses develop.
-
Q2: What is the primary advantage of maintaining blood flow in this model for drug evaluation?
A: Preserving blood flow after thrombus induction is crucial because it enables the evaluation of systemically administered antithrombotic and thrombolytic agents under more physiologically relevant conditions. Drugs can be delivered via the bloodstream to the site of the forming or existing thrombus, mimicking clinical drug distribution and interaction with the circulatory system.
-
Q3: Can this model be used to study thrombus resolution and recanalization?
A: Absolutely. The model's design, which allows for the formation of a stable thrombus in a vessel where blood flow is subsequently maintained, makes it highly suitable for longitudinal studies of thrombus resolution, recanalization processes, and the impact of therapeutic interventions on these outcomes over time.
-
Q4: Is the thrombus composition in this model similar to human DVT?
A: Yes, histological analyses consistently demonstrate that the thrombi formed in the Transient Blood Flow Occlusion-Induced IVC Thrombosis Model are rich in fibrin and red blood cells, closely mirroring the composition of venous thrombi observed in human patients. This similarity enhances the translational relevance of the research findings.
-
Q5: Can this model be adapted for studies on CAT?
A: Indeed, this model is highly adaptable for CAT studies. By utilizing immunocompromised mouse strains, it is possible to engraft human cancer cells, creating a more representative in vivo environment to investigate the complex interplay between cancer and thrombosis, and to test novel therapies for CAT.
-
Q6: What kind of endpoints can be measured using this model?
A: A wide array of endpoints can be measured, including quantitative assessment of thrombus size (weight, length, volume), histological and immunohistochemical analysis of thrombus composition (e.g., fibrin, platelets, red blood cells, inflammatory cells, collagen), vessel patency, and the evaluation of various biochemical markers related to coagulation and inflammation.
Published Data
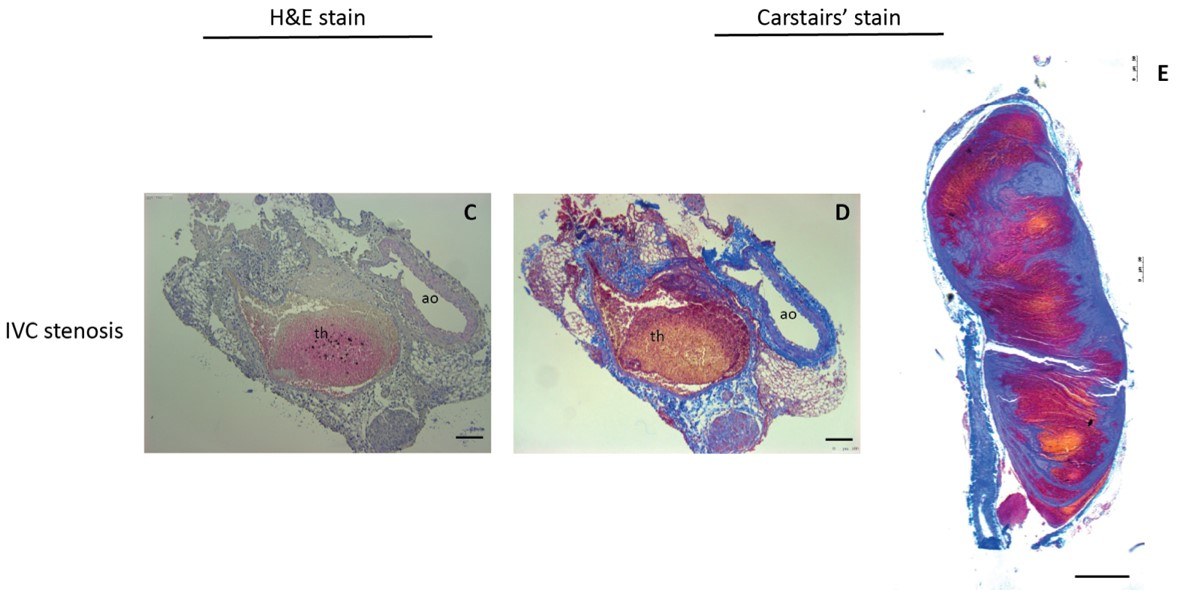 Fig.2 Validation of mouse DVT models induced by IVC ligation.2
Fig.2 Validation of mouse DVT models induced by IVC ligation.2
A study utilized an IVC stenosis model to demonstrate the molecular detection of venous thrombosis in mice using SPECT/CT imaging. This research successfully showed that molecular imaging probes could specifically target and visualize fibrin-rich thrombi formed under conditions of partial blood flow. The findings highlight the model's utility in developing non-invasive diagnostic tools and understanding thrombus characteristics in vivo, offering valuable insights for preclinical drug development and imaging agent validation.
References
- Shah, Mihir S et al. "A Narrative Review on Robotic Surgery as Treatment for Renal Cell Carcinoma with Inferior Vena Cava Thrombus." Journal of clinical medicine vol. 13,5 1308. 26 Feb. 2024 Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/jcm13051308
- Dickhout, Annemiek et al. "Molecular Detection of Venous Thrombosis in Mouse Models Using SPECT/CT." Biomolecules vol. 12,6 829. 13 Jun. 2022. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image. https://doi.org/10.3390/biom12060829
For Research Use Only.
