Gastritis Modeling & Pharmacodynamics Services
Introduction
Gastritis is an inflammation of the stomach lining, which can be classified into acute and chronic forms. Acute gastritis occurs suddenly, often due to factors like infections, excessive alcohol consumption, or the use of certain medications such as nonsteroidal anti-inflammatory drugs (NSAIDs). Chronic gastritis develops gradually over time and may be linked to long-term infections, autoimmune conditions, or prolonged exposure to irritants. One of the most common causes of gastritis is an infection with Helicobacter pylori, a bacterium that damages the stomach lining. Other factors, such as bile reflux and excessive alcohol intake, can also contribute to the condition. Chronic gastritis may eventually lead to complications like gastric ulcers or even gastric cancer if left untreated. Creative Biolabs provides a range of robust models to evaluate the efficacy of treatments for gastritis. These models simulate various forms of the disease, allowing for the comprehensive testing of drugs aimed at reducing inflammation, combating bacterial infections like H. pylori, and targeting other underlying causes of the condition.
Disease Models and Applications
Creative Biolabs offers a diverse selection of well-established rodent models for gastritis research, including both acute and chronic gastritis models. These models are carefully engineered to replicate the pathophysiology of human gastritis, enabling in-depth studies of disease mechanisms and therapeutic interventions. Our gastritis models come with comprehensive evaluations of key parameters such as gastric inflammation, ulcer formation, and histological changes, providing valuable insights for preclinical drug testing. Our expert team of scientists will collaborate with you throughout your research journey, from designing experiments to analyzing and interpreting results, ensuring that you achieve reliable and high-quality outcomes. To explore more about our gastritis models available for preclinical studies, please refer to the links below:
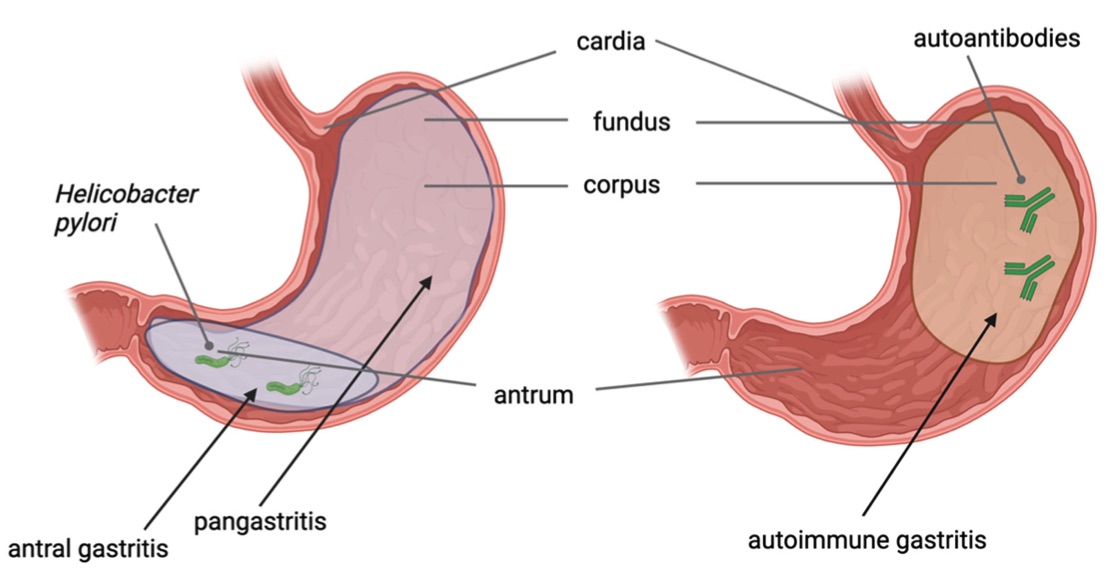 Fig. 1 Distribution of different types of gastric precancerous conditions in the stomach.1
Fig. 1 Distribution of different types of gastric precancerous conditions in the stomach.1
- DOCA & Ethanol & Ammonia Water induced Chronic Atrophic Gastritis Model
- Simulates: The Deoxycholate Sodium (DOC-Na) & Ammonia Water & Indomethacin-Induced Atrophic Gastritis Model simulates chronic atrophic gastritis, a condition characterized by inflammation and damage to the gastric mucosa, leading to glandular atrophy, ulceration, and potential loss of gastric function. This model is induced by the combination of DOC-Na, a bile acid that disrupts the mucosal barrier, ammonia water, which induces further irritation and inflammation, and indomethacin, a nonsteroidal anti-inflammatory drug (NSAID) that inhibits prostaglandin synthesis, exacerbating gastric injury. It closely mimics human conditions of gastritis caused by bile reflux, NSAID use, and environmental toxins.
- Evaluates Drugs: This model is ideal for evaluating drugs aimed at protecting the gastric mucosa from inflammation, damage, and ulceration. It is commonly used to test the efficacy of anti-inflammatory agents, proton pump inhibitors (PPIs), mucosal protectants, and agents that promote gastric healing and reduce oxidative stress. Additionally, it is used to assess therapies designed to prevent gastric ulceration or enhance the healing of damaged gastric tissue caused by NSAID use, bile reflux, and toxic exposure.
Measurements
We offer a variety of measurements for evaluating drug efficacy in rodent gastritis models, utilizing an array of advanced technologies, including but not limited to:
- General observations: body weight, mortality rate, stool consistency, presence of gastrointestinal bleeding, and behavioral changes indicative of discomfort or pain.
- Histopathology: Tissue examination of the gastric mucosa for signs of inflammation, epithelial damage, ulceration, and glandular atrophy.
- Immunohistochemistry: Detection of immune cell infiltration (e.g., T-cells, macrophages) in gastric tissues to assess the inflammatory response and tissue injury.
- Cytokine profiling (e.g., ELISA): Measurement of inflammatory mediators such as TNF-α, IL-6, IL-1β, and other cytokines involved in gastric inflammation.
- Hematology analysis and serum biomarkers: Monitoring of serum markers such as liver enzymes, C-reactive protein (CRP), and gastric enzymes (e.g., pepsinogen, gastric acid secretion).
- Gene/protein expression profiling via RT qPCR and Western blot techniques: Quantification of gene expression related to inflammation, oxidative stress, and gastric mucosal integrity, such as COX-2, iNOS, and NF-κB.
In addition to the established models of gastritis disease, our expertise extends to the development of novel animal models tailored to specific research needs, including models of autoimmune gastritis, Helicobacter pylori-induced gastritis, and drug-induced gastritis. Our scientific team is available to assist in experimental design, model selection, and data analysis, ensuring a customized and effective approach to your project at every stage.
Related Services
Advantages
- Advanced Technology: We utilize cutting-edge techniques, including immunohistochemistry, cytokine profiling, gene/protein expression analysis, and histopathology, to provide in-depth insights into the mechanisms of disease and the efficacy of therapeutic interventions.
- High-Quality Data: Our rigorous quality control processes guarantee reproducible, accurate, and reliable data, which is critical for advancing drug development and preclinical research.
- Fast Turnaround Time: We understand the urgency of your research, so we offer quick response times and efficient study execution to meet your project timelines without compromising on quality.
- Collaborative Approach: We work closely with you throughout your project, from the initial design to the final analysis, ensuring that you receive expert support and guidance at every step.
- Ethical and Reliable: All of our studies are conducted under strict ethical guidelines, with a commitment to the welfare of the animals and adherence to regulatory standards.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What endpoints can be measured in gastritis studies?
A1: Key endpoints include body weight, clinical signs of gastritis, histological examination of gastric tissue, inflammatory cytokine levels, serum biomarkers, and gene/protein expression related to inflammation and gastric injury.
-
Q2: Can the gastritis models be used for drug evaluation?
A2: Yes, our models are specifically designed for evaluating the efficacy of anti-inflammatory drugs, cytoprotective agents, and therapies aimed at promoting gastric healing or reducing mucosal damage.
-
Q3: Do you provide assistance with experimental design?
A3: Absolutely. Our scientific team works closely with you to assist in experimental design, model selection, and the identification of relevant biomarkers, ensuring your research objectives are met efficiently.
-
Q4: Can these models be customized for specific research needs?
A4: Yes, we offer customization of models and experimental protocols to address specific research goals, such as testing particular drug types, varying treatment regimens, or targeting specific mechanisms involved in gastritis.
-
Q5: What types of data analysis and reporting do you provide?
A5: We provide comprehensive data analysis, including statistical analysis, biomarker quantification, histopathological interpretation, and gene/protein expression profiling. Detailed reports are provided, offering insights into the effects of your treatments.
Published Data
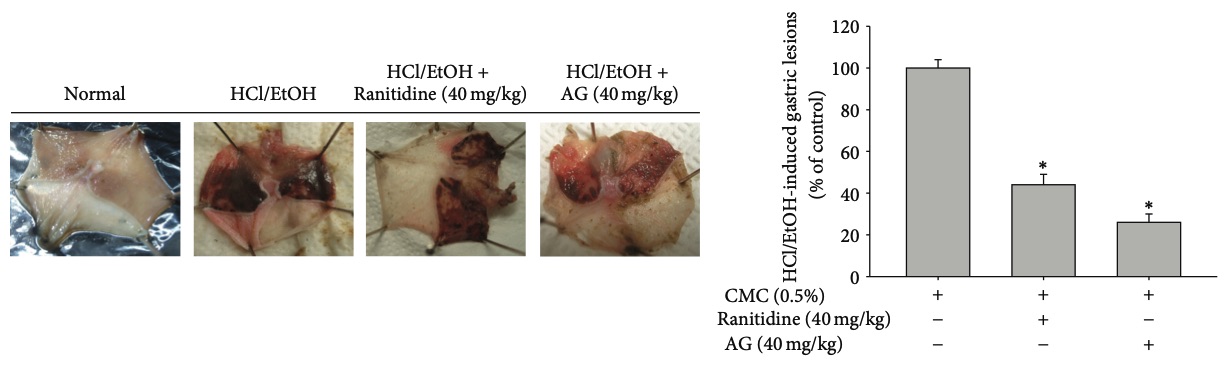 Fig. 2 HCl/EtOH-induced gastritis animal models.2
Fig. 2 HCl/EtOH-induced gastritis animal models.2
The present study investigated the molecular mechanisms of Andrographolide (AG) and its target proteins in relation to anti-inflammatory responses. Mice were orally administered AG or ranitidine for 3 days and then treated with EtOH/HCl to induce gastritis. One hour after treatment, the stomachs were resected, and photographs were taken of the gastric lesions (left panel). The areas of the gastric lesions were measured (right panel). In the EtOH/HCl-induced gastritis model, AG significantly reduced the area of gastric lesions, comparable to the effects of ranitidine (40 mg/kg) and rofecoxib (10 mg/kg), a selective COX-2 inhibitor (data not shown). These results suggest that AG is an orally available anti-inflammatory agent with effects similar to those of established anti-inflammatory treatments.
References
- Romańczyk, Marcin et al. "Non-Invasive Markers for the Detection of Gastric Precancerous Conditions." Cancers vol. 16,12 2254. 18 Jun. 2024, DOI:10.3390/cancers16122254. Distributed under an Open Access license CC BY 4.0, without modification.
- Shen, Ting et al. "AP-1/IRF-3 Targeted Anti-Inflammatory Activity of Andrographolide Isolated from Andrographis paniculata." Evidence-based Complementary and Alternative Medicine: eCAM vol. 2013 (2013): 210736. DOI:10.1155/2013/210736. Distributed under an Open Access license CC BY 4.0. The image was modified by extracting and using only Part C of the original image.
For Research Use Only.
