Low Density Lipoprotein Receptor Deficient (LDLR-/-) Mice Modeling & Pharmacodynamics Service
At Creative Biolabs, we provide a diverse range of well-established rodent models, including the LDLR-/- mouse, to accurately evaluate the efficacy of novel anti-atherosclerotic interventions.
Introduction
Atherosclerosis, a chronic inflammatory disease of the arterial wall, represents a leading cause of cardiovascular morbidity and mortality worldwide. Its complex pathogenesis involves dyslipidemia, inflammation, and cellular dysfunction, culminating in the formation of atherosclerotic plaques. Effective strategies to combat this global health burden necessitate robust and physiologically relevant preclinical models.
Low-Density Lipoprotein Receptor-Deficient Mice Model
The low-density lipoprotein receptor (LDLR) is a crucial cell surface protein responsible for clearing cholesterol-rich LDL particles from the bloodstream. Genetic inactivation of the LDLR gene in mice creates the LDLR-deficient (LDLR-/-) model, which faithfully recapitulates key features of human familial hypercholesterolemia. These mice exhibit significantly elevated plasma LDL-cholesterol levels and spontaneously develop atherosclerotic lesions that closely mimic human plaque morphology and progression. This makes the LDLR-/- model an indispensable tool for investigating the mechanisms of hyperlipidemia-driven atherosclerosis and assessing the therapeutic potential of new compounds.
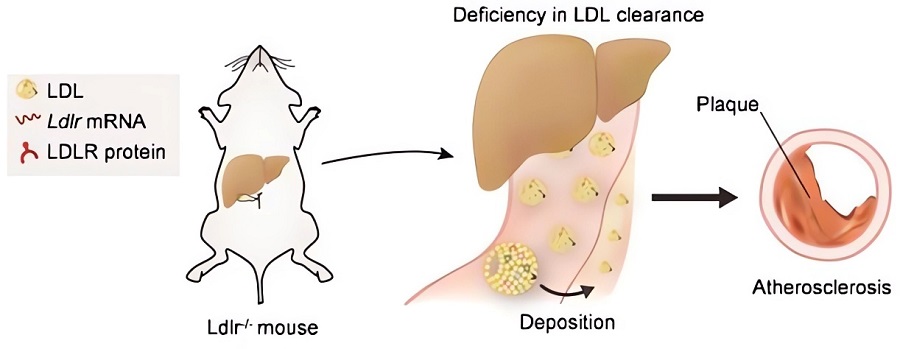 Fig.1 The abnormal lipid metabolism and atherosclerosis in LDLR-/- mice.1,3
Fig.1 The abnormal lipid metabolism and atherosclerosis in LDLR-/- mice.1,3
Model Construction Steps
The construction of the LDLR-/- mouse model typically involves advanced genetic engineering techniques:
01Targeting Vector Design
A specialized DNA construct, known as a targeting vector, is engineered to contain sequences homologous to the LDLR gene, flanking a selectable marker gene that disrupts the LDLR coding sequence.
02Embryonic Stem (ES) Cell Transfection
The targeting vector is introduced into mouse ES cells, where homologous recombination can occur, replacing the functional LDLR gene with the disrupted version.
03Selection and Expansion
ES cells that have successfully undergone homologous recombination are selected using the resistance marker and expanded in culture.
04Chimeric Mouse Generation
These modified ES cells are then injected into early mouse embryos (blastocysts), which are subsequently implanted into surrogate mothers. This results in the birth of chimeric mice, containing cells derived from both the modified ES cells and the host embryo.
05Germline Transmission and Breeding
Chimeric mice are bred with wild-type mice. Offspring that inherit the modified LDLR gene (identified through genotyping) are heterozygous (LDLR+/-). These heterozygotes are then interbred to produce homozygous LDLR-/- mice, which are completely deficient in the LDLR.
Strengths and Limitations
Strengths:
- Physiologically Relevant Lipid Profile: LDLR-/- mice, particularly on an atherogenic diet, develop hypercholesterolemia with a lipid profile that closely resembles dyslipidemic humans, primarily due to elevated LDL-C.
- Spontaneous and Reproducible Atherosclerosis: They spontaneously develop atherosclerotic lesions that are histologically similar to human plaques, with predictable progression in key arterial sites like the aortic root.
- High Responsiveness to Interventions: The model is highly sensitive to dietary changes and pharmacological agents targeting lipid metabolism and inflammation, making it excellent for efficacy testing.
- Versatility for Mechanistic Studies: Allows for detailed investigation of genetic, cellular, and molecular pathways involved in atherosclerosis.
Limitations:
- Cost and Maintenance: Compared to simple diet-induced models in wild-type mice, maintaining a colony of genetically modified mice can be more resource-intensive.
- Species-Specific Differences: While highly relevant, no mouse model perfectly replicates all aspects of human atherosclerosis, particularly complex plaque rupture mechanisms.
Evaluation Platform
Our comprehensive evaluation platform utilizes state-of-the-art biochemical, molecular, cellular, and histopathological techniques, complemented by advanced imaging capabilities, to provide a holistic assessment of atherosclerosis progression and therapeutic efficacy.
Key Test Parameters Include:
- Lipid Profiling: Total cholesterol, LDL-C, HDL-C, triglycerides.
- Inflammatory Markers: Circulating cytokines (e.g., TNF-α, IL-6), chemokines, and acute phase proteins.
- Plaque Burden & Composition: Histological quantification of lesion area, volume, and cellular components (macrophages, smooth muscle cells, collagen, lipid core).
- Vascular Function: Endothelium-dependent and -independent vasodilation.
- Gene and Protein Expression: Analysis of key genes and proteins involved in lipid metabolism, inflammation, and vascular remodeling.
Applications
- Simulated Diseases: Atherosclerosis, hyperlipidemia, familial hypercholesterolemia, and cardiovascular complications associated with metabolic syndrome.
- Evaluated Drugs: Hypolipidemic agents (e.g., statins, PCSK9 inhibitors), anti-inflammatory compounds, anti-thrombotic therapies, and novel compounds targeting vascular health.
- Investigated Treatments: Gene therapies aimed at restoring LDLR function or introducing protective factors, dietary interventions, and lifestyle modifications.
Related Atherosclerosis Models
- ApoE-/- Mice Model
- ApoE*3 Transgenic (E3L) Mice Model
- Fatty Zucker Rats Model
- Carotid Artery Endothelial Denudation Model
- High-Fat-Diet (HFD) & CHOL-Induced Aorta Atherosclerosis Model
- Blood Flow-Induced Arterial Intimal Thickening Model
Our Advantages
- Unrivaled Expertise: Our team comprises seasoned biologists with extensive experience in preclinical atherosclerosis research and LDLR-/- mouse models.
- Customized Study Design: We collaborate closely with clients to develop bespoke study protocols, optimizing experimental design for specific research objectives.
- Comprehensive Phenotyping: We offer a full suite of advanced analytical services, ensuring detailed and accurate characterization of disease progression and therapeutic response.
- State-of-the-Art Facilities: Our vivarium adheres to the highest animal welfare standards, guaranteeing the integrity and reproducibility of all studies.
- Accelerated Research Timelines: Our efficient processes and dedicated resources help expedite your drug discovery and development programs.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is dedicated to providing high-quality preclinical research services utilizing the LDLR-/- mouse model. We are committed to supporting your scientific endeavors and accelerating the translation of your innovative therapies. Contact us today to discuss how our expertise and resources can advance your next cardiovascular research project.
FAQs
-
Q1: How does the LDLR-/- model compare to ApoE-/- mice, another common atherosclerosis model?
A: While both models develop atherosclerosis, the LDLR-/- mouse more closely mirrors the human lipid profile, with elevated LDL. ApoE-/- mice, conversely, accumulate VLDL and chylomicron remnants. The choice between models often depends on the specific lipoprotein pathway or therapeutic target under investigation.
-
Q2: What type of diet is typically recommended for inducing atherosclerosis in LDLR-/- mice?
A: Although LDLR-/- mice develop hypercholesterolemia on a regular chow diet, an atherogenic diet, typically high in fat and cholesterol (e.g., Western diet), is commonly used to accelerate and exacerbate lesion development, leading to more robust and reproducible plaque formation within a shorter timeframe.
-
Q3: Can the LDLR-/- model be effectively utilized for studies focused on plaque regression?
A: Absolutely. The LDLR-/- model is well-suited for investigating plaque regression. Once advanced lesions have developed, researchers can implement therapeutic interventions or dietary changes and then assess the reduction in plaque burden, changes in plaque composition, and improvements in vascular function.
-
Q4: Are there specific genetic backgrounds of LDLR-/- mice that are particularly advantageous for certain studies?
A: Yes, the C57BL/6 background is the most commonly used for LDLR-/- mice due to its susceptibility to atherosclerosis. Specific substrains or engineered variants, such as the C57BL/6J-LdlrHlb301/J, can offer accelerated lesion development, which may be beneficial for studies requiring faster progression or higher plaque burden.
-
Q5: Is it possible to combine the LDLR-/- model with other genetic modifications for more complex research questions?
A: Indeed. The LDLR-/- model can be combined with other genetic modifications (e.g., additional knockouts, transgenics) to create more sophisticated models. This allows researchers to investigate the interplay of multiple genes or pathways in atherosclerosis, providing deeper insights into disease mechanisms.
-
Q6: What kind of data and reports can I anticipate receiving from a typical LDLR-/- study conducted by Creative Biolabs?
A: You can expect comprehensive data packages, including detailed raw data, statistical analyses, high-resolution histological images of atherosclerotic lesions, quantitative measurements of plaque burden and composition, lipid profiles, and inflammatory marker assessments. All findings are presented in a clear, concise, and professionally formatted report.
Published Data
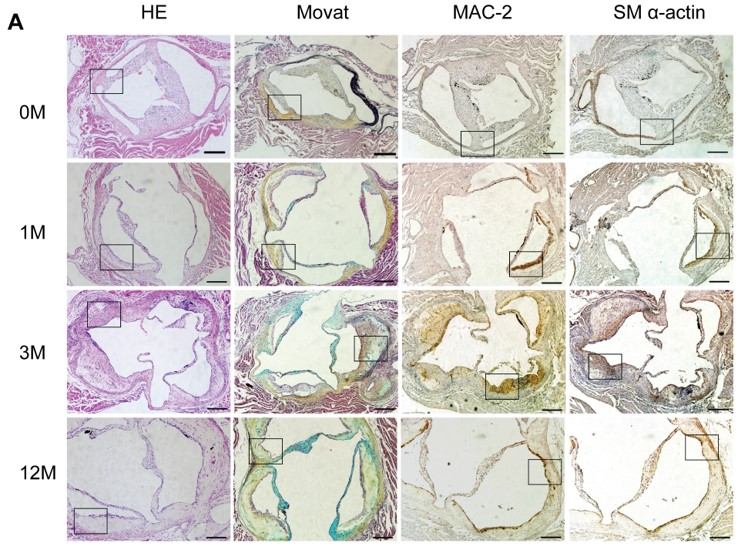 Fig.2 Atherosclerotic lesion development in the aortic sinus of LDLR-/- mice.2,3
Fig.2 Atherosclerotic lesion development in the aortic sinus of LDLR-/- mice.2,3
This study provides a typical case demonstrating the model's utility. In this project, LDLR-/- mice on a high-fat diet exhibited time-dependent and site-selective atherosclerotic lesion development, with initial lesions in the aortic root progressing to the thoracic and abdominal aorta. This study's detailed analysis of lesion progression and metabolic parameters showcases the model's robustness for evaluating therapeutic interventions aimed at mitigating atherosclerosis.
References
- Li, Zhelong et al. "Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model." Theranostics vol. 11,6 2953-2965. 1 Jan. 2021. DOI: 10.7150/thno.49874
- Ma, Yanling et al. "Hyperlipidemia and atherosclerotic lesion development in Ldlr-deficient mice on a long-term high-fat diet." PloS one vol. 7,4 (2012): e35835. https://doi.org/10.1371/journal.pone.0035835
- Distributed under Open Access license CC BY 4.0, without modification. The image was modified by extracting and using only part of the original image.
For Research Use Only.
