Gentamicin induced Acute Renal Failure Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established models for evaluating acute renal failure and testing therapeutic agents aimed at protecting the kidneys. These models, including gentamicin-induced ARF, offer a reliable platform for drug discovery, allowing for detailed assessment of nephrotoxic effects and the efficacy of potential treatments in a controlled setting.
Introduction
Acute renal failure (ARF), also known as acute kidney injury (AKI), is a rapid decline in kidney function characterized by a sudden increase in serum creatinine levels and a reduction in urine output. This condition can result from various causes, including trauma, infection, ischemia, toxins, or drug-induced damage. The kidneys play a vital role in filtering waste products from the blood, maintaining fluid balance, and regulating electrolyte levels. When kidney function is impaired, it can lead to a buildup of harmful substances in the body, electrolyte imbalances, and fluid retention, which can be life-threatening if not addressed promptly. There are several types of ARF, including prerenal (caused by inadequate blood flow to the kidneys), intrinsic (due to direct damage to the kidney tissue), and postrenal (resulting from urinary tract obstructions). Symptoms of ARF include fatigue, decreased urine output, swelling, confusion, and, in severe cases, multi-organ failure. Treatment typically involves addressing the underlying cause, managing fluid and electrolyte balance, and, in some cases, dialysis to support kidney function.
Gentamicin-Induced Acute Renal Failure Model
The gentamicin-induced acute renal failure model is constructed by administering gentamicin to rodents, which leads to nephrotoxic effects, predominantly in the proximal tubules. This model is particularly useful for assessing the pathophysiology of drug-induced kidney damage and for testing potential nephroprotective agents. A major advantage of this model is its ability to simulate human-like kidney injury in a controlled laboratory setting. However, one limitation is that the gentamicin dosage must be carefully monitored to avoid excessive toxicity, which could complicate the analysis of potential therapeutic interventions.
- Simulates: This model simulates drug-induced nephrotoxicity, specifically mimicking the pathophysiology of acute kidney injury (AKI). This model replicates key features of renal damage, such as tubular necrosis, inflammation, and renal dysfunction, making it an ideal tool for studying the effects of nephrotoxic drugs on kidney function.
- Evaluates Drugs: This model is primarily used to evaluate nephroprotective drugs, anti-inflammatory agents, and potential therapies that aim to reduce kidney damage. It is also effective for testing drugs designed to improve renal recovery, prevent tubular damage, and modulate inflammatory responses during kidney injury. The model helps assess both the efficacy and safety of novel treatments aimed at acute kidney injury and related disorders.
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in the gentamicin-induced acute renal failure model, including:- General Observations: body weight, mortality rate, urine output, signs of renal dysfunction (e.g., hematuria, proteinuria).
- Histopathological Analysis: examination of kidney tissue for tubular necrosis, glomerular damage, and interstitial inflammation.
- Serum Biomarkers: creatinine, blood urea nitrogen (BUN), cystatin C, and KIM-1 levels.
- Cytokine Profiling: quantification of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-18 using ELISA.
- Gene/Protein Expression: RT-qPCR and Western blotting to assess markers of renal injury and repair, such as NGAL, Nrf2, and fibronectin.
- Renal Function: Assessment of renal clearance, glomerular filtration rate (GFR), and electrolyte imbalance.
In addition to standard kidney injury markers, we utilize advanced imaging techniques such as MRI and histology to examine tissue structure and kidney function over time. Our team of experts is available to assist with experimental design and interpretation of results, ensuring comprehensive insights into drug efficacy and mechanisms of action.
Related Services
Apart from the gentamicin-induced acute renal failure model, we also offer other methods of inducing acute kidney injury. These alternative models can complement the gentamicin-induced model to provide a broader understanding of kidney damage and therapeutic response.
Our advantages
- Wide Application: Suitable for a broad range of drug testing, from nephrotoxic agents to potential kidney-protective therapies.
- Expertise: Our team provides hands-on support throughout the study, from model selection to data analysis.
- Tailored Solutions: Customizable experimental protocols to meet the unique needs of your research.
- Reliable Results: High reproducibility and consistency in the model, ensuring reliable and valid results for your drug development projects.
- Comprehensive Services: In addition to modeling, we provide histopathological and biomarker analysis, offering a complete service for your study.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What species can be used for this model?
We primarily use rodents such as rats and mice, but can also provide options for larger species based on your research requirements.
-
2. How long does it take to see results in the model?
Typically, kidney damage can be observed within 7 to 10 days after gentamicin administration, with significant recovery or deterioration depending on the drug treatment.
-
3. Can this model be used for testing combination therapies?
Yes, this model is ideal for testing both single-agent and combination therapies aimed at mitigating kidney damage.
-
4. What other models are available for renal disease research?
In addition to the gentamicin model, we offer models for diabetic nephropathy, hypertension-induced kidney damage, and polycystic kidney disease.
-
5. Can you provide custom analysis services?
Yes, we offer custom measurement options and data analysis services to suit specific research needs.
Published Data
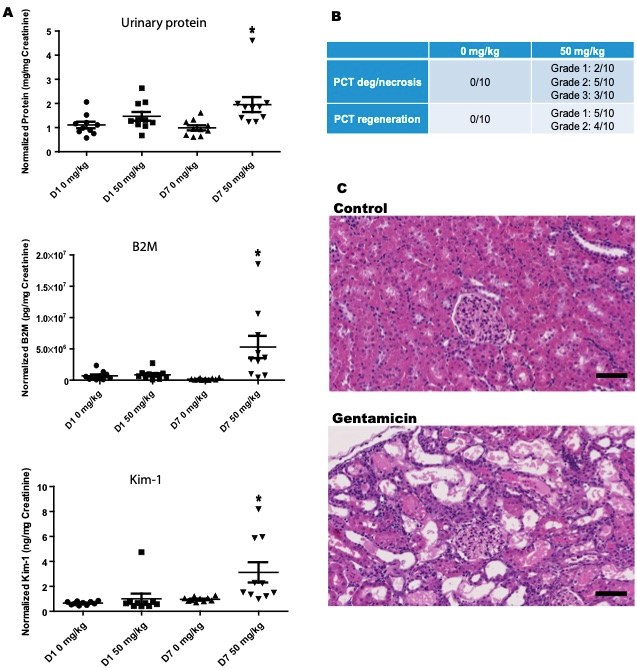 Fig.1 Gentamicin-induced nephrotoxicity in rats.1
Fig.1 Gentamicin-induced nephrotoxicity in rats.1
An experiment was conducted to characterize miRNA changes that could serve as biomarkers for acute renal tubular injury, within the framework of the HESI committee on Biomarkers of Nephrotoxicity. The study utilized a well-established rat model of gentamicin toxicity. Rats were administered subcutaneous injections of gentamicin daily for seven days at various doses, including 0 and 50 mg/kg/day. After 24 hours of dosing, only minimal changes in urine biomarkers were observed. However, after 7 days of treatment, significant increases in urine total protein, Beta-2-Microglobulin, and Kim-1 levels were detected, indicating renal tubular injury (Figure 1A). These urinary biomarker levels showed strong correlation with histopathological findings, which revealed damage to the proximal convoluted tubules (PCT), and, to a lesser extent, the distal convoluted tubules (DCT). The histopathological changes included tubular epithelial degeneration and necrosis, with varying degrees of vacuolar and hyaline degeneration, loss of cellular detail, and detachment from the basement membrane. Cellular debris, as well as cellular and hyaline casts, were found in the tubular lumens (Figure 1B-C). These findings were associated with multifocal, mild tubular epithelial regeneration in the PCT, characterized by basophilic cytoplasm, vesicular nuclei, and occasional mitotic figures. Three rats exhibiting moderate PCT degeneration and necrosis (grade 3) at Day 7 were selected for further miRNA analysis via next-generation sequencing (NGS). Additionally, minimal to mild interstitial mononuclear leukocytic infiltrates were observed. These histological changes were correlated with increased organ weight and macroscopically pale and enlarged kidneys.
Reference
- Nassirpour, Rounak et al. "Identification of tubular injury microRNA biomarkers in urine: comparison of next-generation sequencing and qPCR-based profiling platforms." BMC Genomics vol. 15,1 485. 18 Jun. 2014. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1186/1471-2164-15-485
For Research Use Only.
