Hi-Affi™ Humanized CSF1R Immune Checkpoint Knockin Mouse Model
Immune checkpoint receptors are vital molecules for optimized adjusting immune responses. The tumor-permissive and immunosuppressive characteristics of tumor-associated macrophages (TAM) have aroused interest in therapeutically targeting these cells. Particularly, CSF1R-mediated signaling is critical for the differentiation and survival of the mononuclear phagocyte system and macrophages. Since CSF1R is also overexpressed in many cancers and on tumor-associated macrophages, CSF1R inhibitors have been widely studied for years as a potential treatment for cancer or inflammatory diseases. As a leading innovator in drug discovery, Creative Biolabs has developed a Hi-Affi™ “humanized” mouse model platform for therapeutic antibody development and evaluation. Especially, we now provide the humanized CSF1R knock-in mouse models and professional technical support for your novel antibody preclinical efficacy studies.
CSF1R Immune Checkpoint Pathway
Colony-stimulating factor 1 receptor (CSF1R), also known as macrophage colony-stimulating factor receptor (M-CSFR) and CD115 (Cluster of Differentiation 115), belongs to the type III protein tyrosine kinase receptor family and is a single pass type I membrane protein encoded by the CSF1R gene (also known as c-FMS) in human. CSF1R acts as the receptor for colony stimulating factor 1 (CSF1), which is a cytokine that controls the production, differentiation, and function of macrophages. Macrophages are known as a highly plastic cell type that adjusts to the specific stromal environment present in malignant tumors, characterized by low oxygen pressure, tissue necrosis, and high concentrations of lactate and pyruvate.
The Significance of CSF1R Immune Checkpoint Pathway
Binding of CSF1 or the more recently identified ligand, IL-34 could induce homodimerization of CSF1R and subsequent activation of signaling through a process of oligomerization and trans-phosphorylation. As the intra-tumoral presence of CSF1R+ macrophages correlates with poor survival in numerous tumor types, targeting CSF1R signaling in tumor-promoting TAM (tumor-associated macrophage) proved to be an attractive strategy to eliminate or repolarize these cells. In addition, both CSF1R and its ligand colony stimulating factor 1 play an important role in the development of the mammary gland and may be involved in the process of mammary gland carcinogenesis.
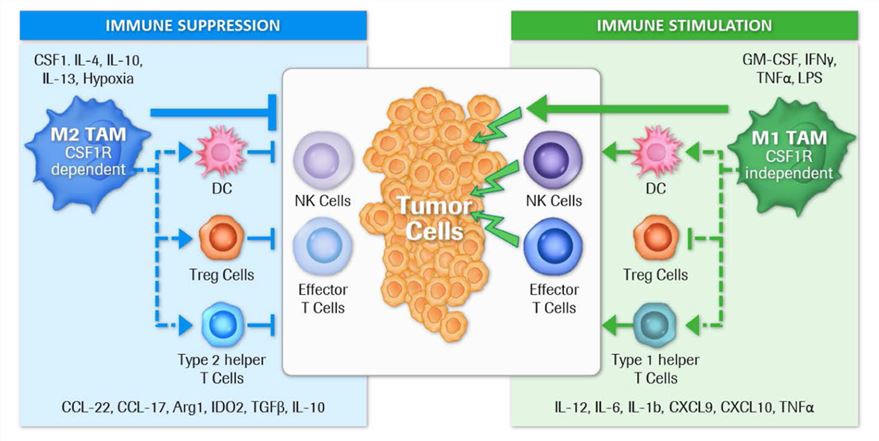 Fig.1. Direct and indirect regulation of immune suppression or stimulation by tumor-associated macrophage subtypes. (Cannarile, et al., 2017)1, 2
Fig.1. Direct and indirect regulation of immune suppression or stimulation by tumor-associated macrophage subtypes. (Cannarile, et al., 2017)1, 2
Development of Humanized CSF1R Immune Checkpoint Knock-In Mice
A range of small molecules and mAbs (monoclonal antibodies) targeting CSF1R or its ligand CSF1 are in clinical development both as monotherapy and in combination with standard treatment modalities such as chemotherapy as well as other cancer-immunotherapy approaches. Several studies have demonstrated that CSF1R inhibitors are a promising new class of immune-modulatory drugs. Scientific understanding of macrophage and CSF1R biology is developing rapidly, and more data from preclinical animal models investigating CSF1R-directed therapies will surely help to further optimize associated cancer immunotherapies. Scientists in Creative Biolabs successfully established a series of Hi-Affi™ humanized mouse models for in vivo efficacy evaluation of novel drugs, including CSF1R immune checkpoint KI mouse generated via Cas9-mediated homologous recombination. Please feel free to contact us for more detailed information.
Creative Biolabs also offers other various Humanized Mouse Models you may be interested in:
References
- Cannarile, M. A.; et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. Journal for Immunotherapy of Cancer. 2017, 5(1):53.
- under Open Access license CC BY 4.0, without modification.
For Research Use Only.
