Creative Biolabs provides ligand binding tests that determine how strongly and precisely an antibody binds to its target immunological checkpoint. To assess binding affinity, they employ methods such as Surface Plasmon Resonance (SPR) and Bio-Layer Interferometry (BLI).
Immune Checkpoint Binding & Interaction Assay Services
The Evolving Role of Immune Checkpoint Assays in Gene Therapy-Enhanced Cancer Immunotherapy
Immune checkpoints are critical regulators of immune responses, which tumor cells often exploit to evade detection and destruction by the immune system. The current landscape of cancer therapy has been revolutionized by immunotherapies, particularly immune checkpoint inhibitors (ICIs). However, a subset of patients still experiences resistance or limited benefits. Gene therapy offers a promising avenue to enhance anti-tumor immunity by modulating the tumor microenvironment or directly influencing immune checkpoint pathways. For instance, combining gene-targeted therapies with ICIs has shown synergistic effects in preclinical and clinical studies, improving response rates in various cancers, including glioblastoma. Developing robust immune checkpoint binding and interaction assays is therefore essential to identify novel targets, understand resistance mechanisms, and precisely characterize the efficacy of new therapeutic combinations, paving the way for more effective and durable cancer treatments.
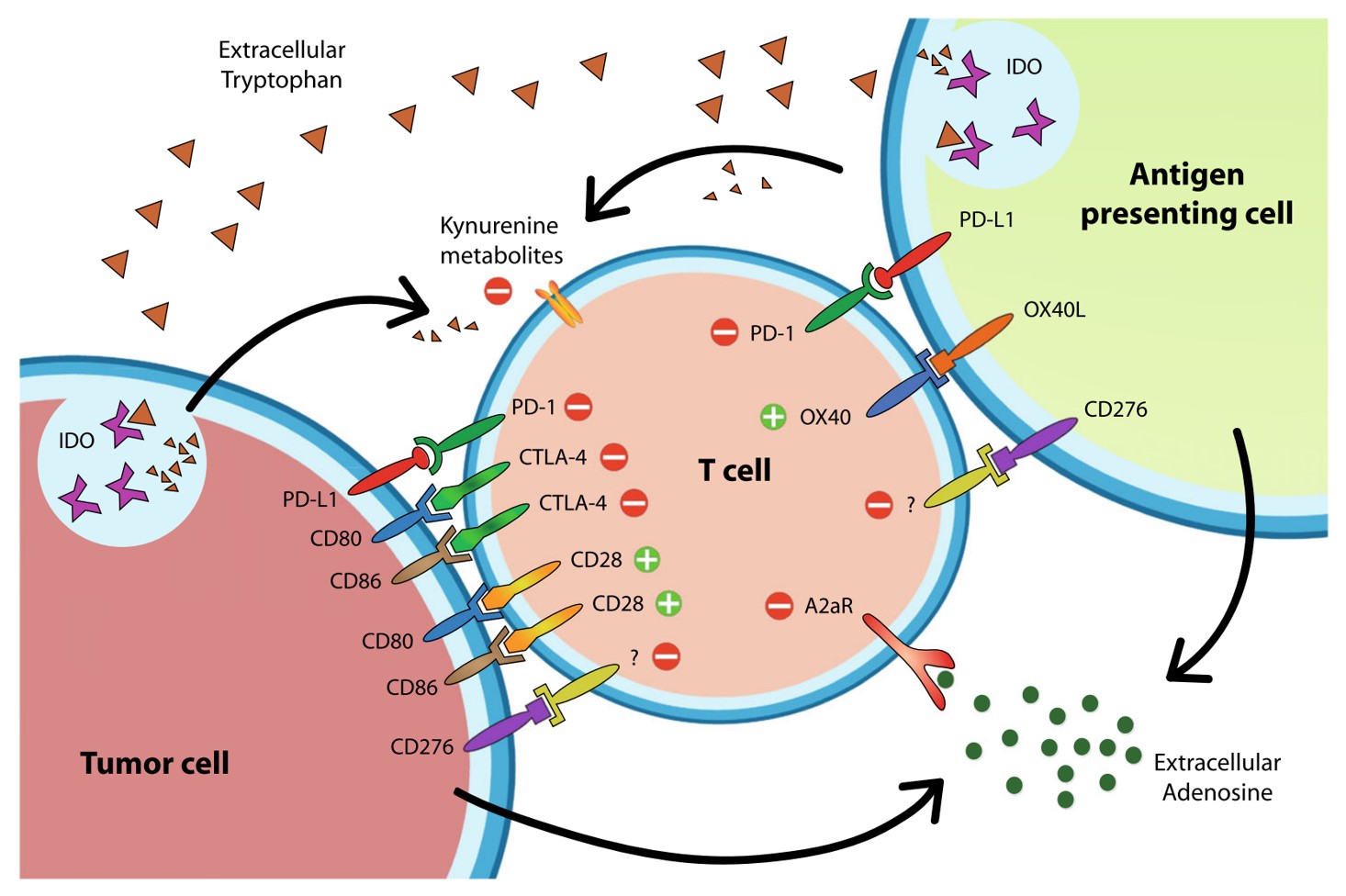 Fig.1 The expression and interactions of immune checkpoints on cells.1
Fig.1 The expression and interactions of immune checkpoints on cells.1
Immune Checkpoint Binding & Interaction Assays at Creative Biolabs
Creative Biolabs offers comprehensive solutions for characterizing antibody-immune checkpoint interactions, providing crucial insights for your drug discovery and development projects. Our services are designed to deliver specific, actionable data to accelerate your research, typically involving a collaborative project consultation, rigorous binding and cell-based functional assays, and detailed data analysis, with a usual timeframe ranging from 4 to 8 weeks depending on project complexity.
Workflow: From Sample to Solution
- Project Consultation & Design: Our expert team collaborates with you to understand your specific research objectives, target immune checkpoints, and desired outcomes. We then design a customized assay strategy tailored to your project's unique requirements.
- Protein & Ligand Preparation: We ensure the availability of high-quality, biologically active immune checkpoint proteins and their corresponding ligands, either through our in-house recombinant protein expression and purification services or by sourcing validated materials.
- Binding Assay Execution: We conduct rigorous ligand binding tests employing modern biophysical methods. These assays precisely determine the binding affinity, dynamics (association and dissociation rates), and specificity of your antibody candidates to immune checkpoint targets.
- Cell-based Functional Assays: To validate the physiological relevance and mechanism of action, we integrate various cell-based assays. This includes cell-based binding assays using flow cytometry or ELISA, and highly sensitive Reporter Gene Assays. These assays assess how your antibodies modulate immune cell activation, cytokine release (e.g., IL-2, IL-8), or other functional readouts, providing critical insights into their therapeutic potential.
- Data Analysis & Comprehensive Reporting: All raw data undergo thorough analysis and interpretation by our experienced scientists. You will receive a detailed, comprehensive report summarizing the experimental methods, raw data, processed results, and insightful conclusions, ready for your regulatory submissions or further research.
Our Service Packages
Creative Biolabs offers a variety of specific assays for comprehensive immune checkpoint analysis:
Cell-Based Binding Assay
To thoroughly validate the mechanism of action for an antibody, Creative Biolabs uses a range of cell-based assays. Immune checkpoint antibodies work in two main ways: by blocking inhibitory pathways (antagonists) or by enhancing activating pathways (agonists). Their assays are designed to test both of these functions.
Primary Cell Assay
In this type of assay, Creative Biolabs uses primary lymphocytes as effector cells and custom or well-known tumor cell lines as targets. The results are measured using physiologically relevant biomarkers, such as the release of cytokines like IL-2 or IL-8. They also use ELISA or flow cytometry to analyze cell-binding properties.
Reporter Gene Assays
Creative Biolabs also uses specially engineered cell lines that stably express specific receptors instead of primary cells. In these assays, the activation of the effector cells is measured by monitoring luciferase activity. By comparing results with and without a test antibody, they can determine the antibody's potential to interact with checkpoint pathways and restore immune function.
Available Targets for Reporter Assays
We focus on a full range of checkpoint receptors, including but not limited to:
| Activating Receptors | Inhibitory Receptors |
|---|---|
| GITR | PD-1 Receptor |
| 4-1BB Receptor | TIGIT Receptor |
| CD40 Receptor | CTLA-4 Receptor |
| OX40 | LAG3 |
| CD27 | TIM-3 Receptor |
| VEGF | BTLA |
| HVEM | TNFα |
| SIPRα |
Service Highlights
- Customized Experiments.
- Efficient Procedure.
- Extensive Expertise.
- One-stop Services.
Popular Products at Creative Biolabs
Creative Biolabs provides an extensive range of kits to assist in the development of cancer immunotherapies. Our immune checkpoint-related products include inhibitory screening kits and combination bioassay kits. Explore our offerings below to find the appropriate product for your research demands.
FAQs
-
Q1: What types of immune checkpoints can Creative Biolabs analyze?
A1: We offer comprehensive analysis for a full range of activating and inhibitory immune checkpoint receptors, including but not limited to PD-1, PD-L1, CTLA-4, LAG-3, TIM-3, and TIGIT. Our assays are highly adaptable to various targets.
-
Q2: How do your assays ensure the quality and reliability of results?
A2: We maintain stringent quality control measures throughout the assay process, from using high-quality recombinant proteins to employing clinical-grade reference antibodies as positive controls. Our advanced platforms (SPR, BLI, reporter gene systems) and experienced scientists ensure accurate and reproducible data.
-
Q3: Can Creative Biolabs customize the assays for specific project needs?
A3: Absolutely. We understand that each project is unique. We offer highly customized experimental designs, allowing you to tailor the assays to your specific antibody candidates, target immune checkpoints, and research objectives.
-
Q4: What kind of data will I receive from the Immune Checkpoint Binding & Interaction Assays?
A4: You will receive comprehensive data packages, including detailed binding kinetics (affinity, association, and dissociation rates), functional readouts from cell-based assays (e.g., cytokine release, reporter gene activity), and a thorough project report with our expert analysis and conclusions.
-
Q5: How do these assays compare to other methods for studying immune checkpoint interactions?
A5: Our integrated approach combines biophysical binding assays with cell-based functional assays, providing a more complete picture of your antibody's mechanism of action compared to single-method analyses. This comprehensive data is crucial for robust therapeutic development.
Contact Us
Creative Biolabs is your trusted partner in advancing immunotherapeutic research. Our immune checkpoint binding & interaction assays provide the precise and comprehensive data you need to accelerate your drug discovery pipeline and bring innovative treatments to patients. For more detailed information, please feel free to contact us or directly send us an inquiry.
Reference
- Wierstra, Peter et al. "Tracers for non-invasive radionuclide imaging of immune checkpoint expression in cancer." EJNMMI radiopharmacy and chemistry vol. 4,1 29. 6 Nov. 2019, doi:10.1186/s41181-019-0078-z. Distributed under an Open Access License CC BY 4.0, without modification.
For Research Use Only.
