Bispecific Immune Checkpoint targeted Reporter Gene Assay Service
Targeting Bi-Specific Immune Checkpoint Brings Great Potential to Cancer Immunotherapy.
Immune checkpoint blockade (ICB) with antibodies has yielded sustained clinical benefits across various cancer types. Amongst, monoclonal antibodies have demonstrated significant success in this field; however, their action is limited to a single target, thereby restricting their therapeutic potential. Emergingly, bispecific antibodies represent a groundbreaking class of drugs capable of engaging two distinct immune checkpoint proteins, whether on the same or different antigens. This dual-targeting capability not only enhances their binding affinity and tumor eradication effectiveness but also aids in lowering both development and clinical trial expenses.
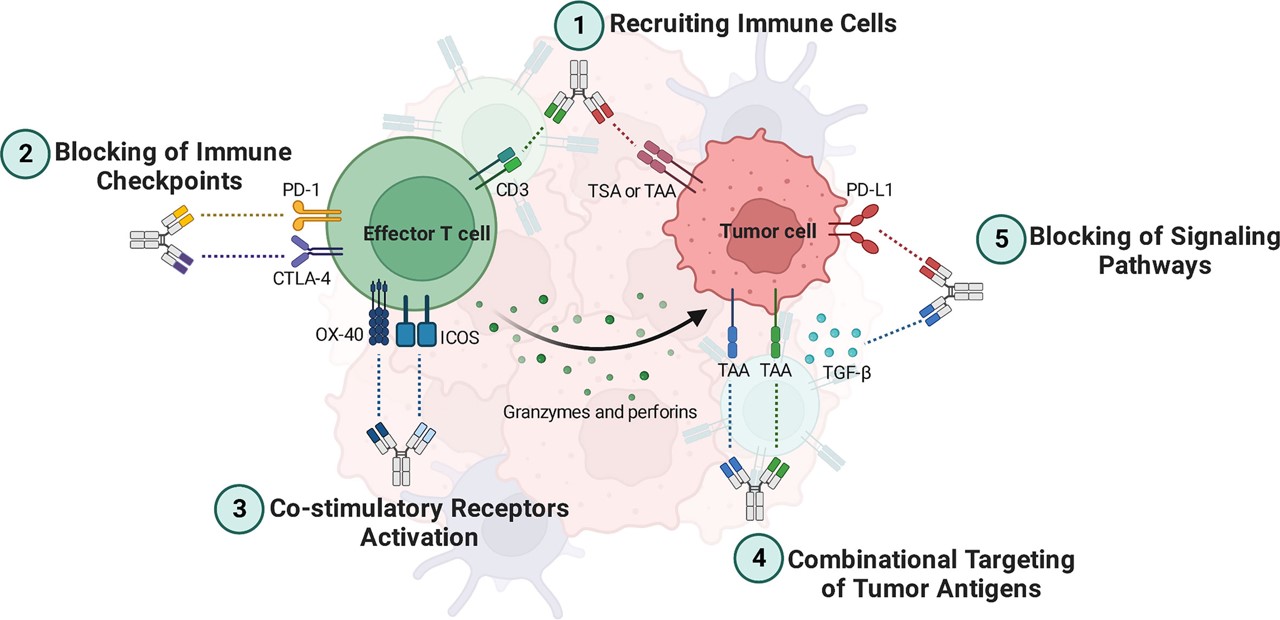 Fig.1 Mechanisms of bi-specific antibodies.1,3
Fig.1 Mechanisms of bi-specific antibodies.1,3
Bi-specificity Immune Checkpoint-targeted Reporter Gene Assay at Creative Biolabs
Cell-based reporter gene assays have revolutionized the way researchers evaluate the function of therapies targeting bi-specific immune checkpoints. Compared to traditional primary cell-based assays, reporter gene assays are notably more efficient, consistent, and reproducible. Creative Biolabs has pioneered an advanced reporter gene assay specifically designed to target bispecific immune checkpoints to serve for targeted therapeutics screening or evaluation.
Our bispecific immune checkpoint targeted reporter gene assays are designed to evaluate the effects of candidate compound treatment on engineered cell lines expressing bispecific immune checkpoints integrated with a reporter system to screen or evaluate candidate compounds. The reporter gene response's intensity indicates the candidate's effector function, offering clear and quantifiable efficacy readouts. Meanwhile, our versatile sample loading capacity allows for simultaneous multi-sample measurement. Furthermore, we offer custom cell line development for customers wishing to conduct research in your own lab. At Creative Biolabs, our expert team provides custom solutions designed to meet unique customer requirements, guaranteeing exceptional quality and swift delivery.
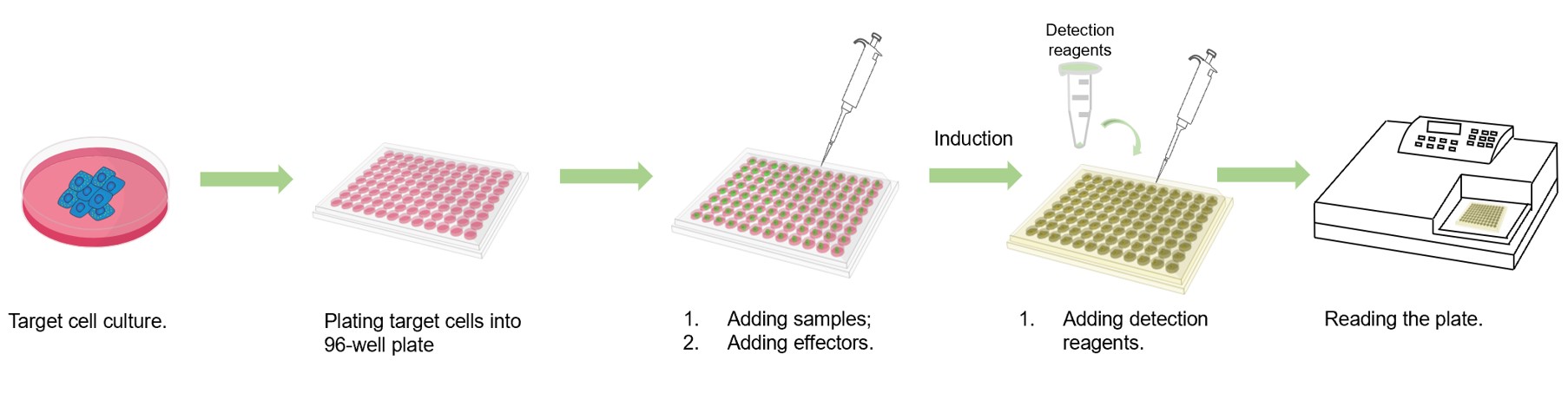 Fig.2 Workflow of our assay.
Fig.2 Workflow of our assay.
Our High-click Bi-specificity Immune Checkpoint-targeted Reporter Gene Assay
With the increasing focus on therapeutics targeting bispecific immune checkpoints, Creative Biolabs offers specialized reporter gene assays tailored for various bispecific immune checkpoint combinations, aiming to facilitate the development of targeted therapies with our comprehensive services. The following are several hot-click bi-specificity immune checkpoint-targeted reporter gene assays:
- PD-1/TIGIT-targeted Reporter Gene Assay
- PD-1/LAG3-targeted Reporter Gene Assay
- PD-1/41BB-targeted Reporter Gene Assay
- PD-1/CTLA-4-targeted Reporter Gene Assay
Candidate Classification
Our versatile assay system is designed to accommodate a broad spectrum of samples, including antibodies and diverse biologics.
- Antibodies
- Small molecular
Benefits for You
 Fig.3 Key features of our assay.
Fig.3 Key features of our assay.
Representative Data
| Summary: | To investigate the efficacy of anti-PD-L1/CD40L BsAbFP, a dual-binding fusion protein targeting human CD40 and PD-L1, this study developed a specialized reporter gene bioassay. The assay utilized engineered CHO cells to serve as surrogate antigen-presenting cells (APCs) expressing both a T cell receptor activator and PD-L1, Jurkat cells modified to express PD-1 along with an NFAT-luciferase reporter acting as effector T cells, and Raji cells with an NFκB-luciferase reporter that naturally express CD40, serving as accessory B cells. This innovative assay simultaneously engages the PD-1/PD-L1 and CD40/CD40L signaling pathways to evaluate the functionality of anti-PD-L1/CD40L BsAbFP. |
| Results: |
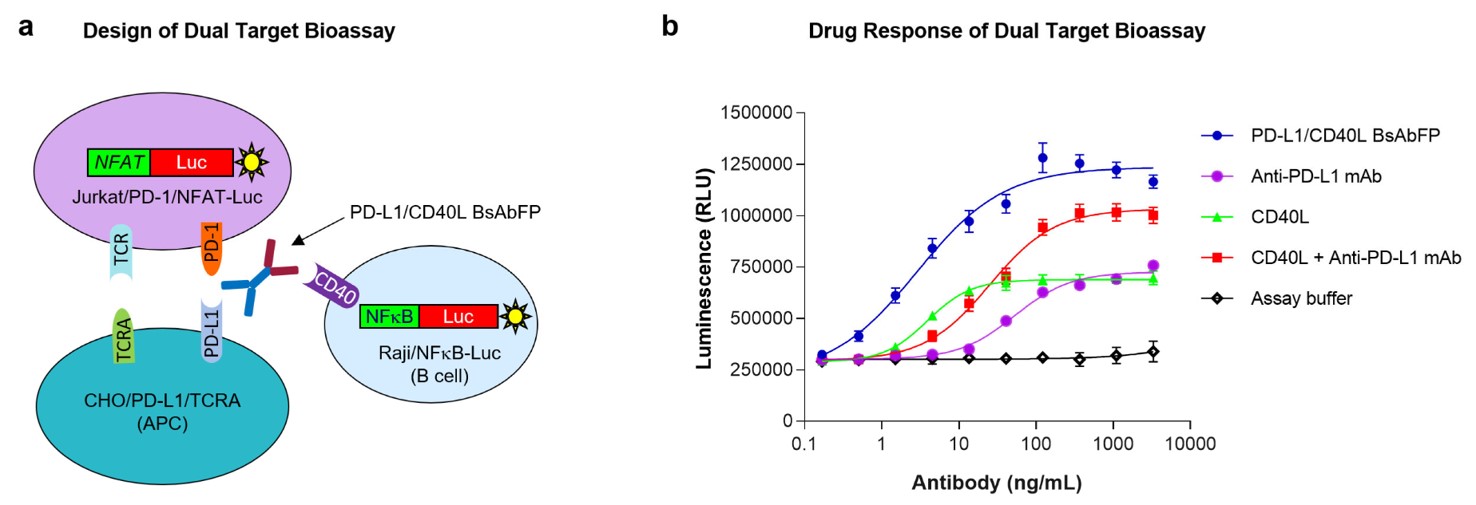 Fig.4 Anti-PD-L1/CD40L BsAbFP evaluation through a dual target reporter gene assay.2, 3 |
Frequently Asked Question
Q1: What are the requirements for shipping samples?
A1: To ensure the optimal performance of our bi-specific immune checkpoint-targeted reporter gene assay, we need customers to supply basic information about their candidates. This includes details such as molecular weight, targets, purity, concentration, endotoxin level, and any relevant characteristics that may impact the assay outcomes. Upon receiving your inquiry, our team of experts will evaluate your project needs and offer bespoke solutions. Upon your approval, we will initiate the implementation plan following the receipt of your samples.
If you want to know more about our bi-specific immune checkpoint-targeted reporter gene assay, please get in touch with us at your convenience.
References
- Farhangnia, Pooya, et al. "Bispecific antibodies targeting CTLA-4: game-changer troopers in cancer immunotherapy." Frontiers in Immunology 14 (2023): 1155778.
- Pandey, Madhu S., et al. "Simultaneous inhibition of PD-1 and stimulation of CD40 signaling pathways by anti-PD-L1/CD40L bispecific fusion protein synergistically activate target and effector cells." International Journal of Molecular Sciences 22.21 (2021): 11302.
- under Open Access License CC BY 4.0, without modification.
For Research Use Only.
