Graft Versus Host Disease (GVHD) Modeling & Pharmacodynamics Services
Creative Biolabs offers a range of well-established Graft Versus Host Disease (GVHD) models, enabling the evaluation of potential drug candidates. These models simulate human disease progression and immune responses, providing a reliable platform for testing therapeutic interventions and advancing GVHD research.
Introduction
Graft Versus Host Disease (GVHD) is a life-threatening condition that typically occurs after allogeneic stem cell or organ transplantation. It arises when the donor's immune cells recognize the recipient's tissues as foreign and mount an immune response against them. GVHD primarily affects the skin, liver, and gastrointestinal system, and it can be categorized into acute or chronic forms. Acute GVHD usually develops within the first 100 days after transplantation and manifests as rash, diarrhea, and liver dysfunction. Chronic GVHD, which may appear later, involves long-term complications such as fibrosis and immune system dysregulation. Acute GVHD is characterized by an inflammatory response, whereas chronic GVHD can lead to tissue scarring and functional organ impairment. The severity of GVHD is influenced by the degree of histocompatibility mismatch between donor and recipient, and the use of immunosuppressive therapies is a critical part of treatment.
Graft versus host disease (GVHD) Models and Applications
Creative Biolabs offers a wide range of well-established rodent models for Graft Versus Host Disease (GVHD) research. These models are designed to simulate the immune-mediated condition that occurs after an allogeneic stem cell or bone marrow transplant, where donor immune cells attack the host tissues. GVHD is a major complication in stem cell transplantation, affecting various organs such as the skin, liver, and gastrointestinal tract. Our GVHD models are carefully designed to mimic the acute and chronic phases of the disease, enabling the evaluation of potential therapeutic candidates. These models are extensively used to study the mechanisms of GVHD and to assess the efficacy of treatments aimed at preventing or mitigating immune rejection, reducing organ damage, and improving survival rates. Our team of experienced scientists will work alongside you from experimental design through data analysis, ensuring reliable and high-quality results. To learn more about the GVHD models available for preclinical research, please explore the links below:
| GVHD Model | Simulates | Evaluates Drugs | Animal species |
| Acute GVHD Model (Skin & Gut Targeting) | Immune-mediated tissue damage in the skin, liver, and gastrointestinal tract following stem cell or bone marrow transplant. | Immunosuppressive agents (e.g., corticosteroids, cyclosporine), biologics targeting T-cell activation (e.g., anti-CD3), therapies for organ-specific GVHD (e.g., anti-TNFα), and cytokine inhibitors. | Mouse |
| Chronic GVHD Model | Chronic immune-mediated tissue fibrosis, inflammation, and dysfunction affecting multiple organs over a prolonged period. | Fibrosis-modifying drugs, JAK inhibitors, immune modulators, therapies targeting chronic inflammation, and steroid-sparing agents. | Mouse |
Evaluation Platform
- Animals: Mouse.
-
Measurements
We offer a variety of methods to evaluate drug efficacy in Graft Versus Host Disease (GVHD) models, employing advanced techniques such as:- General Observations: Mortality rate, body weight changes, clinical scoring of symptoms (rash, diarrhea).
- Histological Analysis: Tissue damage and infiltration of immune cells in target organs (skin, liver, gut).
- Immunohistochemistry: Analysis of immune cell populations (T-cells, dendritic cells, macrophages) in affected tissues.
- Cytokine Profiling: ELISA to measure key cytokines like IL-6, TNF-α, and IFN-γ, reflecting immune activation.
- Hematology and Serum Analysis: Liver enzymes, white blood cell counts, and other biomarkers indicating organ damage and immune response.
- Gene Expression Profiling: RT-qPCR to assess gene expression related to immune function, inflammation, and fibrosis.
Our advantages
- Customizable Model Designs: We provide tailored models suited for specific research needs.
- Comprehensive Evaluation: Our platform includes a wide range of endpoints and measurement techniques for in-depth analysis.
- Expert Consultation: Our experienced team assists with study design, data analysis, and interpretation, ensuring high-quality results.
- Advanced Research Tools: Access to cutting-edge technologies like cytokine profiling, histological analysis, and gene expression studies.
- Rigorous Quality Control: Our models are validated and refined to ensure reproducibility and reliability.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What strains of animals are used for GVHD models?
We use a variety of murine strains, with the most common being C57BL/6 or BALB/c, depending on the specific nature of the study.
-
2. How long does the GVHD model study last?
The study duration can vary, but typically lasts between 3 to 6 weeks, depending on the disease progression and intervention strategies.
-
3. Can you simulate both acute and chronic GVHD?
Yes, we offer models for both acute and chronic forms of GVHD, with different disease timelines and pathologies.
-
4. What types of drugs can be tested in this model?
The model can be used to test a wide range of therapeutics, including immunosuppressive drugs, anti-inflammatory agents, and those targeting fibrosis.
-
5. Can the model be adapted for human therapeutic studies?
While there are species-specific differences, this model provides valuable insights and can be adapted to assess potential human therapies.
Published Data
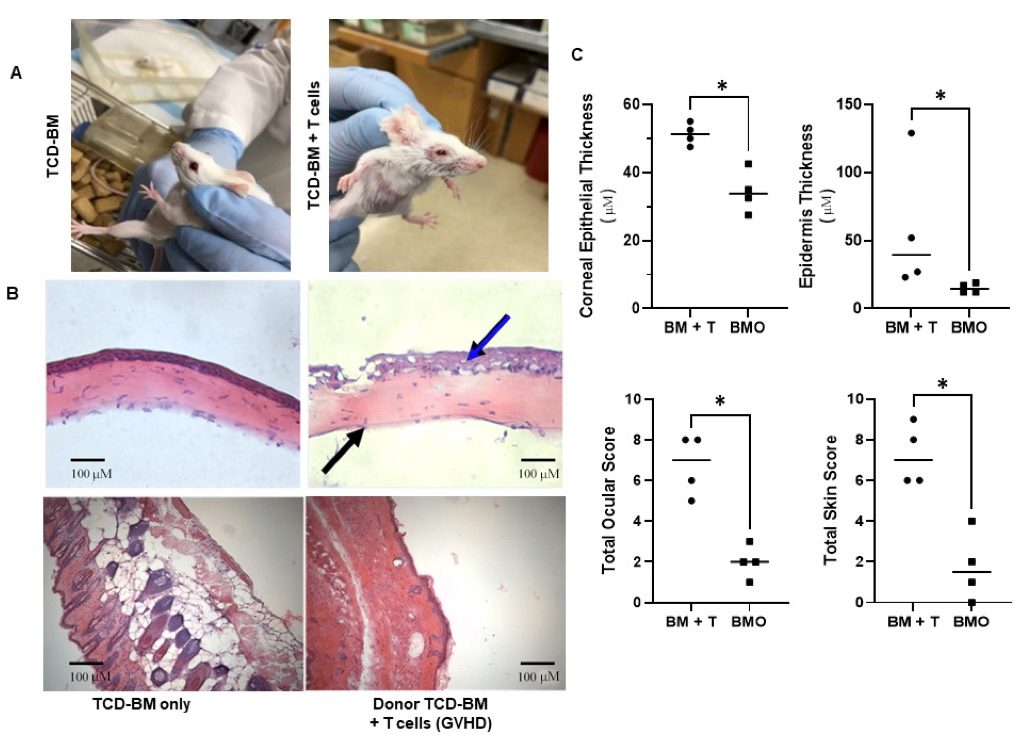 Fig. 1 Development of ocular and skin GVHD following HSCT in an MHC-matched donor/recipient strain combination.1
Fig. 1 Development of ocular and skin GVHD following HSCT in an MHC-matched donor/recipient strain combination.1
Recipients of donor bone marrow (BM) and T cells exhibited notable eyelid swelling, corneal keratopathy, and skin abnormalities, including ruffling, alopecia, and scabbing (Figure 1A). Histological analysis revealed epithelial corneal thickening (blue arrow) and corneal stromal thinning (black arrow) in animals developing GVHD, which were absent in control mice transplanted with BM alone (Figure 1B). Examination of the skin (Figure 1B, lower panels) demonstrated thickening of both the epidermal and dermal layers, extensive collagen deposition infiltrating the underlying adipose tissue, and loss of hair follicles in T cell recipients undergoing GVHD, whereas BM-only controls did not show these alterations. A scoring system was applied to quantify corneal epithelial thickening and stromal thinning, as detailed in the Methods section. Analysis of these scores revealed significant increases in corneal and skin epithelial thickening in mice affected by GVHD (Figure 1C, upper panels), as well as elevated total ocular and skin pathology scores compared with controls (Figure 1C, lower panels).
Reference
- Levy, Robert B et al. "Analyses and Correlation of Pathologic and Ocular Cutaneous Changes in Murine Graft versus Host Disease." International Journal of Molecular Sciences vol. 23,1 184. 24 Dec. 2021. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/ijms23010184
For Research Use Only.
