Peritonitis Modeling & Pharmacodynamics Services
Creative Biolabs offers a variety of well-established peritonitis models to assess the efficacy of potential drugs, providing a valuable platform for researchers focused on treating this life-threatening condition.
Introduction
Peritonitis is an acute inflammation of the peritoneum, the thin tissue that lines the abdominal wall and covers most abdominal organs. It is most commonly caused by bacterial infections, typically following a ruptured appendix, diverticulitis, or trauma to the abdominal area. The infection can also arise from chemical irritants, such as bile or gastric acid. Symptoms of peritonitis include severe abdominal pain, fever, nausea, and vomiting, and if left untreated, it can quickly lead to sepsis, organ failure, and even death. There are two main types of peritonitis: primary peritonitis, which occurs without an obvious source of infection, and secondary peritonitis, which results from an underlying condition like a perforated organ. In severe cases, the infection can spread rapidly, necessitating immediate medical intervention, including surgery and broad-spectrum antibiotics. Because of its critical nature and rapid progression, peritonitis presents significant challenges for clinical management, making effective therapeutic development essential. Animal models of peritonitis are crucial for understanding disease mechanisms, testing new treatments, and developing strategies to prevent complications such as sepsis. These models closely simulate the pathophysiological changes observed in human peritonitis, enabling accurate preclinical testing of potential drugs.
Peritonitis Models and Applications
Creative Biolabs offers a comprehensive range of well-established rodent models for peritonitis. These models are carefully designed to replicate the pathophysiology of peritoneal inflammation and infection seen in humans, making them ideal for evaluating a wide array of therapeutic candidates. We provide detailed assessments of disease progression, immune response, and the efficacy of potential treatments, ensuring robust data for drug development. Our experienced scientific team will collaborate with you at every stage, from experimental design to data analysis, to ensure high-quality, reliable results. For more information on the peritonitis models available for preclinical research, please explore the links below:
| Models | Simulates | Evaluates Drugs | Animal species |
| MSU induced Peritonitis Model | Gouty peritonitis caused by monosodium urate (MSU) crystals | Anti-inflammatory drugs (e.g., NSAIDs, corticosteroids), Immunomodulators, Pain management therapies, Sepsis and organ failure prevention treatments | Rat, Mouse |
| Zymosan induced Peritonitis Model | Acute inflammatory response from zymosan (yeast-derived) mimicking bacterial infection | Anti-inflammatory drugs, Antibiotics, Immunomodulators, Pain management therapies, Sepsis prevention treatments | Rat, Mouse |
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in rodent peritonitis models, utilizing advanced technologies, including but not limited to:- General observations: body temperature, abdominal swelling, signs of infection (e.g., redness, discharge), and survival rates.
- Histopathology: Tissue examination for peritoneal inflammation, immune cell infiltration (e.g., neutrophils, macrophages), and tissue damage.
- Cytokine profiling (e.g., ELISA): Levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in serum and peritoneal fluid.
- Bacterial load quantification: Measurement of bacterial count in peritoneal fluid, blood, and organs.
- Biomarker analysis: Serum markers including C-reactive protein (CRP) and procalcitonin (PCT).
- Immunohistochemistry: Immune cell infiltration and tissue damage in peritoneal and abdominal organs.
Our expertise extends to developing customized animal models tailored to specific research needs, with support throughout experimental design, model selection, and data analysis.
Our advantages
- Customizable protocols: Tailor peritonitis models to meet your specific research needs.
- Experienced scientific support: Guidance through every stage of experimental design, execution, and data interpretation.
- Comprehensive assessment: A broad range of measurements for assessing drug efficacy and disease progression.
- Wide therapeutic scope: Evaluate drugs for diverse treatment options, from antibiotics to anti-inflammatory and immunomodulatory therapies.
- Precise control over disease induction: Ensure reliable results with optimized infection protocols and controlled disease progression.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What types of peritonitis models do you offer?
We provide both bacterial and chemical models, including those induced by E. coli, Klebsiella, and carrageenan, tailored to simulate both primary and secondary peritonitis.
-
2. Can you customize the model for specific research goals?
Yes, we offer flexible protocols to meet the specific needs of your research, whether it's for evaluating drug efficacy, studying disease mechanisms, or investigating novel therapies.
-
3. What kind of drugs can be tested using your models?
Our models are designed to evaluate a broad spectrum of drugs, including antibiotics, anti-inflammatory agents, immunomodulators, and drugs for sepsis prevention and management.
-
4. What are the key endpoints of this model?
Key endpoints include survival rate, infection resolution, cytokine levels, immune cell infiltration, and organ pathology.
-
5. How do you ensure model reliability and reproducibility?
Our models are meticulously standardized, and our team provides continuous support to ensure the accuracy and reproducibility of results.
Published Data
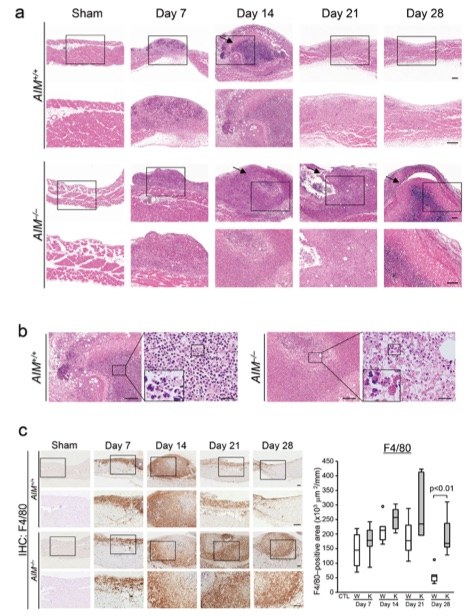 Fig. 1 Morphological changes in the zymosan induced peritonitis model.1
Fig. 1 Morphological changes in the zymosan induced peritonitis model.1
The experiment demonstrated significant inflammatory cell infiltration and necrosis in AIM-deficient mice, which persisted for up to 4 weeks. In contrast, these changes were resolved by 3 weeks post-disease induction in wild-type mice. Light microscopic findings (Figure 1A) revealed necrosis accompanied by intense neutrophilic infiltration and karyorrhexis. The insets in the highly magnified images (Figure 1B) highlight karyorrhexis, a degenerative process characterized by the fragmentation of the condensed nucleus and the irregular distribution of chromatin within the cytoplasm. Additionally, expression of F4/80-positive macrophages was observed in the zymosan induced peritonitis model (Figure 1C). Macrophage infiltration continued for up to 4 weeks in AIM-deficient mice, whereas it significantly decreased by 3 weeks in wild-type mice after disease induction.
Reference
- Tomita, Takako et al. "Apoptosis inhibitor of macrophage ameliorates fungus induced peritoneal injury model in mice." Scientific Reports vol. 7,1 6450. 25 Jul. 2017. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1038/s41598-017-06824-6
For Research Use Only.
