Allergic Asthma Modeling & Pharmacodynamics Services
Introduction
Allergic asthma is the most common asthma phenotype, strongly linked to exposure to environmental allergens like dust mites, pollen, and pet dander, as well as genetic predisposition. Currently, it affects around 300 million people worldwide, with its incidence continuing to rise annually. Allergic asthma pathophysiology involves IgE-mediated type I hypersensitivity, leading to the overactivation of Th2-type immune cells, group 2 Innate Lymphoid Cells (ILC2s), mast cells, and eosinophils, which then release cytokines and activate signaling pathways, such as Interleukin-33/ST2 and OX40/OX40L, ultimately resulting in allergic manifestations. Presently, effective intervention strategies to prevent the onset of respiratory asthma and early-childhood allergen sensitization remain inadequate. To address this gap, Creative Biolabs has leveraged its extensive experience in pharmacological efficacy to develop a suite of allergic asthma models that possess clear characteristics. These models replicate clinical symptoms and serve as essential tools for advancing preclinical evaluation of new drugs, including small molecules, biopharmaceuticals, gene therapies, vaccines, and cell therapies.
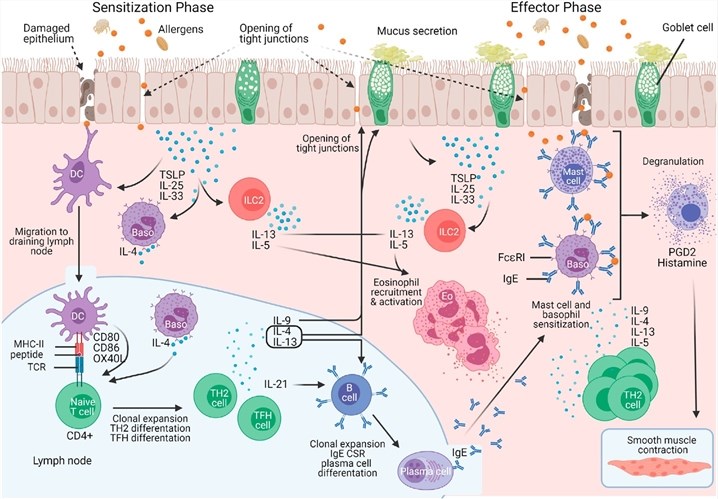 Fig.1 Immune mechanism of allergic asthma.1
Fig.1 Immune mechanism of allergic asthma.1
Available Allergic Asthma Models
At present, we have mainly established four models for studying the asthma pathophysiology and testing potential therapeutics, as follows:
| Allergic Asthma Models | Related Disease | Relevant Drug Evaluation | Animal Species |
| Ovalbumin-Induced Asthma Model | Classic, high-IgE, Th2-driven allergic asthma. | Efficacy testing of Anti-inflammatory drugs, Biologics, and Corticosteroids (e.g., Dexamethasone). | Mouse, Rat, Guinea Pig, Rabbit, NHPs |
| HDM-Induced Allergic Asthma Model | The most clinically relevant asthma triggered by a major environmental aeroallergen. | Evaluating novel therapeutics for asthma triggered by environmental allergens, especially those targeting HDM-specific immune responses. | Mouse, Rat, NHPs |
| MC903 & OVA-Induced Asthma Model | Comorbidity of Atopic Dermatitis (AD) and Asthma (Atopic March), TSLP-mediated inflammation. | Assessing drugs targeting the skin-airway inflammatory axis and inhibitors of epithelial cytokine pathways like TSLP. | Mouse |
| OVA & HDM-Induced Asthma Model | Complex or Severe/refractory Allergic Asthma, Multi-allergen sensitization, Mixed inflammation. | Evaluation of drugs for refractory or atypical inflammatory phenotypes (e.g., neutrophilic asthma). | Mouse |
Evaluation Platform
At Creative Biolabs, we harness a leading-edge technological infrastructure to deliver rigorous endpoint analyses of drug efficacy, providing an unparalleled foundation for the success of your research projects. Beyond basic general clinical observations, the assessment encompasses:
- Immune and Inflammatory Indexes Detection: Humoral immunity is detected by measuring the levels of IgE and cytokines in serum or bronchoalveolar lavage fluid (BALF), and cellular immunity is detected through airway inflammatory cell differential counting and phenotypic analysis of immune cells in lung tissue.
- Lung Function and Airway Reactivity Detection: Airway hyperresponsiveness (AHR) and basic pulmonary ventilation function are assessed using a pulmonary function test system.
- Pathological Histology Evaluation: Lung tissue sections are subjected to various staining techniques, including Hematoxylin and Eosin (HE) staining, Periodic acid-Schiff/Alcian blue (PAS/AB) staining, Masson's trichrome staining, and immunohistochemical staining, among others. Additionally, a semi-quantitative scoring system may be employed to assess the extent of tissue damage.
- Clinical Phenotype and Symptom Simulation Detection: Though detecting the cough reflex sensitivity, airway mucus clearance function, and oxidative stress markers to evaluate the concordance of model clinical and physiological phenotypes with human asthma.
- New Detection Technologies: This encompasses various advanced technologies and novel methodologies, such as organoids, and Organ-on-a-chip (OoC), in vivo imaging techniques, metabolomics, and proteomics.
Applications
- Disease Simulation: By mimicking human conditions such as allergic, environmental, and complex atopic asthma subtypes, these preclinical models accurately reproduce the key pathological features of the disease, including airway hyperresponsiveness (AHR), eosinophilic and/or neutrophilic inflammation, mucus hypersecretion, and airway remodeling.
- Drug Evaluation: The models provide a quantifiable platform for Pharmacodynamics (PD) studies, enabling the assessment of efficacy and safety for novel small molecules (e.g., anti-inflammatory agents), biologics (e.g., anti-IL-5, anti-IL-13, anti-TSLP antibodies), and gene therapies.
- Therapy Advancement & Mechanistic Insight: They also elucidate fundamental molecular mechanisms (e.g., the role of Th2 cells, ILC2s, and epithelial alarmins like TSLP/IL-33), validate novel diagnostic/prognostic biomarkers (e.g., serum IgE, BALF cytokines), and explore the link between skin and lung immunity (Atopic March).
Our advantages
- Professional model development capabilities: We have established diverse asthma animal models that encompass various pathogenic mechanisms to meet specific client needs for model specificity.
- High reproducibility and credibility data: We also implement standardized operating procedures (SOPs) to ensure model reproducibility, experimental data traceable and repeatable, and conduct a full-process review of experimental design, data collection, and result analysis to enhance data credibility.
- Customized capabilities: We possess the capability to develop customized models based on client requirements, such as specific drug targets and mechanisms of action.
- Advanced detection and analysis techniques: We combine advanced multi-dimensional detection platforms, including molecular and cell biology, imaging techniques, immunology, pathology, and high-throughput screening, to meet clients' stringent requirements for data delivery.
- Rich project experience and interdisciplinary team: We have successfully completed numerous projects involving acute asthma models, accumulating significant industry experience that aids in rapid drug evaluation. Our team comprises experts in respiratory medicine, biologists, and statistical analysis specialists, ensuring both the scientific validity of R&D and data reliability.
- One-stop service: We offer a comprehensive range of disease models, spanning multiple system disorders, such as cardiovascular, tumor, and metabolic diseases. This integrated approach also provides drug target screening and toxicity analysis, thereby minimizing time spent on cross-platform communication.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: How to select an appropriate animal for pharmacodynamics evaluation?
A: The choice of the model depends on whether the focus is on specific immune pathways or a broader inflammatory response, guiding the selection of a single or combined model. For predominantly Th2-driven responses, the OVA model is suitable; for more complex immune backgrounds, the HDM or combined models might be preferred. The selected model should closely mimic the patient's condition to enhance the translational value of the data obtained.
-
Q: What is the significance of these models in clinical drug development?
A: These models allow for an in-depth understanding of how drugs modulate immune responses and airway inflammation, shedding light on potential molecular mechanisms. They help in identifying the optimal dosing regimen and predicting clinical efficacy. Initial safety data can be gathered, which is crucial for designing clinical trials.
-
Q: What are the benefits of the OVA & HDM-Induced Asthma Model?
A: Utilizing both allergens, this model can trigger various immune pathways, reflecting a more complex pathological state. It mimics the intense immune reactions patients may experience in environments with multiple allergens, aiding in the evaluation of drug efficacy in real-world scenarios. It offers a multi-faceted approach to assess a drug's impact on inflammation, airway remodeling, and other disease markers.
-
Q: How to ensure the success rate of the model?
A: First, we conduct a pilot experiment before the formal experiment, detect IgE levels and AHR, and expand the sample size after reaching the standard. Second, we perform stringent quality control; for example, each batch of experiments includes a positive group to verify the responsiveness of the model.
Published Data
Myeloid-specific deletion of ARG1 does not affect inflammatory cell infiltration numbers or early lung remodeling in OVA-induced allergic asthma in female mice, suggesting that ARG1's regulation of asthma pathology is limited to the molecular level of inflammatory signaling, rather than cellular/tissue morphological changes.
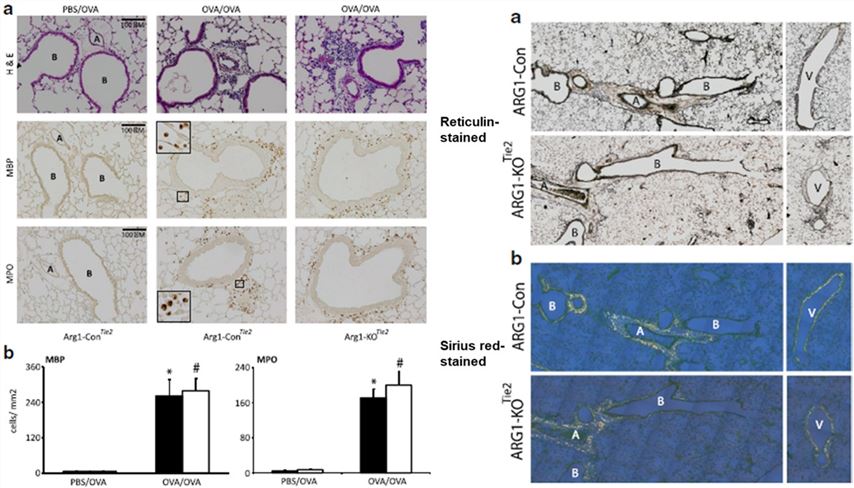 Fig.2 Effect of Arg1 ablation in the lung on the prevalence of inflammatory cells in the lungs and lung remodeling.2
Fig.2 Effect of Arg1 ablation in the lung on the prevalence of inflammatory cells in the lungs and lung remodeling.2
References
- Komlósi, Zsolt I et al. "Cellular and molecular mechanisms of allergic asthma." Molecular aspects of medicine vol. 85 (2022): 100995. https://doi.org/10.1016/j.mam.2021.100995. Distributed under Open Access license CC BY 4.0, without modification.
- Cloots, Roy H E et al. "Arginase 1 deletion in myeloid cells affects the inflammatory response in allergic asthma, but not lung mechanics, in female mice." BMC pulmonary medicine vol. 17,1 158. https://doi.org/10.1186/s12890-017-0490-7. Distributed under Open Access license CC BY 4.0, with modification.
For Research Use Only.
