Psoriasis Modeling & Pharmacodynamics Services
Creative Biolabs offers a range of well-established and customizable models to evaluate the efficacy of psoriasis treatments. These models mimic key features of human psoriasis, enabling detailed studies of therapeutic interventions.
Introduction
Psoriasis is a chronic autoimmune skin disorder characterized by the rapid overproduction of skin cells, leading to thick, scaly plaques. The disease typically affects the skin, but it can also impact the joints (psoriatic arthritis) and other organs. Psoriasis is driven by an overactive immune response, particularly involving T-cells, which trigger inflammatory cytokine release and disrupt normal skin regeneration. This results in the hallmark symptoms of erythema, scaling, and pruritus. Psoriasis can vary in severity, from localized patches to widespread involvement. The condition is also associated with a higher risk of other diseases, including cardiovascular disease, due to systemic inflammation. Currently, treatments focus on managing symptoms, controlling inflammation, and preventing flare-ups. Topical therapies, phototherapy, and systemic biologics are commonly used, but more effective and targeted therapies are still being developed.
Psoriasis Models and Applications
Creative Biolabs offers a comprehensive range of rodent models for studying psoriasis. These models are meticulously designed to simulate key features of human psoriasis, including inflammation, skin lesions, and immune system activation. They are accompanied by thorough evaluations of various clinical and histopathological parameters, enabling the accurate assessment of therapeutic candidates during the preclinical phase. Our experienced team of scientists will support you through all stages of your project, from experimental design to data interpretation, ensuring reliable and high-quality results. To learn more about the psoriasis models available for preclinical research, please explore the links below:
| Model | Simulates Disease | Evaluates Drugs | Animal species |
| Imiquimod (IMQ) induced Psoriasis Rodent Models | Induces inflammation, epidermal hyperproliferation, and skin lesions like human psoriasis. | Anti-inflammatory drugs, biologics targeting IL-17, TNF inhibitors, and topical therapies. | Rat, Mouse |
| IL-23 induced Psoriasis Mouse Model | Activates the IL-23/Th17 pathway, which plays a critical role in psoriasis development. | IL-23 inhibitors, immune-modulating drugs, and therapies targeting Th17 cells. | Mouse |
| Diethylstilbestrol induced Mouse Vaginal Epithelial Mitosis Model | Causes skin hyperproliferation, resembling the epidermal thickening seen in psoriasis. | Anti-proliferative agents, epidermal growth factor inhibitors, and compounds targeting skin regeneration. | Rat, Mouse |
| Mouse Tail Psoriasis Model | Induces local inflammation and thickening of the skin on the tail, mimicking psoriasis plaques. | Topical treatments, cytokine blockers, and immunosuppressants targeting local inflammation. | Mouse |
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a range of measurements to evaluate drug efficacy in psoriasis models, utilizing advanced technologies, including but not limited to:- General Observations: Skin lesion severity, erythema, scaling, and body weight.
- Histopathology: Epidermal thickness, skin inflammation, and immune cell infiltration in skin tissues.
- Immunohistochemistry: Infiltration of immune cells (e.g., T-cells, dendritic cells) and the expression of inflammatory cytokines in the skin.
- Cytokine Profiling (e.g., ELISA): Expression levels of pro-inflammatory cytokines such as TNF-α, IL-6, IL-17, and IL-23.
- Gene/Protein Expression Profiling: Quantification of biomarkers associated with psoriasis pathogenesis (e.g., IL-17, keratin 16) via RT-qPCR and Western blot techniques.
- Clinical Scoring Systems: Psoriasis Area and Severity Index (PASI), erythema, and scaling scores to evaluate therapeutic responses.
In addition to the established psoriasis models, our expertise extends to developing novel models tailored to specific research needs, guided by literature and prior studies. Our scientific team is available to assist in experimental design, model selection, and data analysis, ensuring a customized and effective approach to your project at every stage.
Our advantages
- Expertise in Psoriasis Models: Our models are specifically designed to replicate the key aspects of human psoriasis, ensuring relevance to your research.
- Comprehensive Service: From model selection to data analysis, we offer full support at every stage of your project.
- Tailored Solutions: We provide custom experimental designs to address specific research questions and drug evaluation needs.
- Advanced Technologies: Utilization of cutting-edge methods like immunohistochemistry, cytokine profiling, and gene/protein expression analysis to provide comprehensive results.
- Reliable Results: Our models have been validated in numerous studies, ensuring high-quality and reproducible data for your psoriasis research.
- Collaborative Approach: Our team works closely with you, providing expertise in experimental design, data interpretation, and drug evaluation to optimize your research outcomes.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What are the primary advantages of using the IMQ induced psoriasis model?
The IMQ induced model closely mimics the key features of human psoriasis, including inflammation, epidermal hyperproliferation, and scaling, making it ideal for evaluating systemic and topical therapies.
-
2. How long does it take for psoriasis symptoms to develop in the IL-23 induced model?
Psoriasis-like symptoms typically develop within 7-10 days following induction in the IL-23 model, allowing for timely evaluation of therapeutic candidates.
-
3. Can the psoriasis models be used to study psoriatic arthritis?
While our psoriasis models primarily focus on skin inflammation, they can be adapted for joint involvement studies, particularly in evaluating the systemic effects of treatments.
-
4. Are there any age or gender-specific considerations when selecting a psoriasis model?
Psoriasis models can be used in both male and female rodents, though age-specific models may be selected based on the specific research focus, such as treatment efficacy at different disease stages.
-
5. Do you offer support for drug development with these models?
Yes, our team provides comprehensive support for drug development, including assistance with experimental design, data analysis, and interpreting the results to guide your drug development process.
Published Data
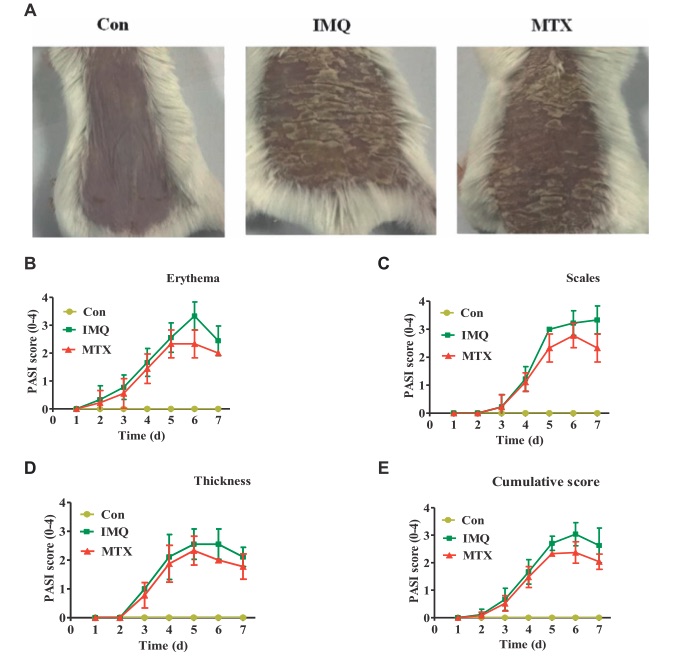 Fig. 1 MTX treatment ameliorated IMQ induced skin lesions. 1
Fig. 1 MTX treatment ameliorated IMQ induced skin lesions. 1
Morphological observations were conducted to assess the effect of MTX on the psoriatic mouse model induced by IMQ. Compared to the control group, IMQ induced skin lesions exhibited typical features such as scaling, erythema, and thickening. These pathological changes were significantly reduced following 7 days of MTX treatment (Figure 1A). The control group showed no significant changes in PASI scores, while the model group displayed progressively worsening skin lesions after IMQ administration. In contrast, mice treated with MTX exhibited less severe scaling, milder erythema, and reduced thickening. The control group maintained a PASI score of 0, whereas the PASI score in the IMQ induced psoriasis group increased from the third day onward due to severe inflammation. The parameters of thickness, erythema, and scaling continued to rise, reaching their peak on days five, six, and seven, respectively. However, these parameters significantly improved after MTX treatment, with PASI scores notably lower compared to the IMQ-treated group (Figures 1B–E).
Reference
- Zong, Jiaxin et al. "Serum Metabolomic Profiling Reveals the Amelioration Effect of Methotrexate on Imiquimod induced Psoriasis in Mouse." Frontiers in Pharmacology vol. 11 558629. 19 Nov. 2020. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fphar.2020.558629
For Research Use Only.
