Experimental Autoimmune Neuritis (EAN) Modeling & Pharmacodynamics Services
Creative Biolabs offers a range of well-established EAN models to evaluate the efficacy of therapeutic candidates targeting autoimmune neuropathies. These models enable researchers to assess the impact of novel drugs aimed at preventing or mitigating nerve damage, reducing inflammation, and promoting nerve regeneration during preclinical studies.
Introduction
Experimental Autoimmune Neuritis (EAN) is an animal model that replicates the features of Guillain-Barré Syndrome (GBS), a rare and severe autoimmune disorder characterized by peripheral nerve damage. GBS occurs when the body's immune system mistakenly attacks the peripheral nerves, leading to muscle weakness, sensory disturbances, and, in severe cases, paralysis. EAN is induced by immunizing animals with myelin proteins, often combined with an adjuvant, to provoke an autoimmune response that leads to inflammatory infiltration, demyelination, and axonal damage in the peripheral nervous system. This model closely mimics the clinical and histopathological characteristics of GBS, making it a powerful tool for studying the disease mechanisms and evaluating potential treatments. EAN is instrumental in research focused on understanding the immune response in neuroinflammatory diseases and offers insights into nerve damage and repair processes.
EAN Models and Applications
Creative Biolabs offers a range of well-established rodent models for experimental autoimmune neuritis (EAN). These models are meticulously designed to simulate Guillain-Barré Syndrome (GBS), a severe autoimmune disorder where the body's immune system attacks the peripheral nerves. The model is induced by immunizing rats with specific peptides (such as P0180-199), leading to inflammation, demyelination, and axonal damage in the peripheral nervous system. Our models provide invaluable tools for studying the underlying mechanisms of autoimmune neuropathies and evaluating potential therapies targeting nerve inflammation, immune responses, and myelin repair. Whether you're focusing on immunomodulatory drugs, neuroprotective agents, or therapies aimed at promoting nerve regeneration, our EAN models offer robust platforms for preclinical testing. Our team of expert scientists will collaborate with you at every stage of your research, from experimental design to data interpretation, ensuring reliable and high-quality results. To learn more about our EAN models available for preclinical research, please explore the links below:
| EAN Models | Simulates | Evaluates Drugs | Animal species |
| P0180-199 Peptide induced EAN Rat Model | Autoimmune-mediated peripheral nerve damage, leading to motor weakness, sensory loss, and paralysis, mimicking Guillain-Barré Syndrome (GBS). | Immunosuppressive agents (e.g., corticosteroids), anti-inflammatory drugs (e.g., TNF-α inhibitors), T-cell inhibitors, neuroprotective therapies, and myelin repair-promoting drugs. | Rat, Mouse |
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in EAN models, utilizing advanced technologies, including but not limited to:- General Observations: Clinical scores based on motor activity, muscle strength, and signs of paralysis or ataxia.
- Histopathology: Assessment of demyelination, axonal damage, and inflammation in peripheral nerves (e.g., sciatic nerve, spinal cord).
- Immunohistochemistry: Detection of immune cell infiltration (e.g., T-cells, macrophages) in affected nerves.
- Cytokine Profiling (e.g., ELISA): Expression levels of inflammatory cytokines such as TNF-α, IL-6, IL-17, and IL-1β in serum and tissues.
- Neurophysiological Testing: Measurement of nerve conduction velocity and compound muscle action potential (CMAP) to assess functional nerve impairment.
- Gene/Protein Expression Profiling: RT-qPCR and Western blot to evaluate biomarkers of inflammation, nerve injury, and remyelination.
- Motor Function Analysis: Rotarod and grip strength tests to assess motor coordination and muscle strength.
In addition to our well-established models, we specialize in developing novel animal models tailored to specific research needs, providing expert guidance on experimental design, model selection, and data analysis to ensure the highest quality results.
Our advantages
- Customized Models: Tailored models to replicate specific aspects of autoimmune neuropathy, including acute and chronic phases of disease.
- Comprehensive Evaluation: Multi-dimensional approach combining clinical observation, histopathological analysis, and neurophysiological measurements.
- Expert Support: Collaborative assistance from our scientific team in experimental design, data interpretation, and model selection.
- Flexible Applications: Suitable for testing a wide range of drugs, including immunosuppressive agents, regenerative therapies, and biologics.
- Proven Reliability: Well-established and reproducible results, providing confidence in the validity of preclinical data.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What are the key symptoms observed in the EAN model?
Symptoms include muscle weakness, loss of coordination, ataxia, and paralysis, typically starting in the hind limbs and progressing to more severe forms of motor dysfunction.
-
2. How long does the EAN model take to show symptoms?
Symptoms typically appear within 10-14 days post-immunization, with peak disease severity occurring around 21 days, depending on the model and the specific protocol used.
-
3. Can this model be used to evaluate treatments for chronic neuropathies?
Yes, the model can be modified to represent both acute and chronic phases of autoimmune neuropathies, allowing for long-term drug testing and evaluation of therapies that promote nerve regeneration and recovery.
-
4. What types of drugs can be evaluated in this model?
The model is ideal for evaluating immunosuppressive drugs, anti-inflammatory therapies, biologics targeting cytokines, T-cell inhibitors, and drugs aimed at promoting nerve repair and remyelination.
-
5. Are there any limitations to the EAN model?
While the model closely mimics the features of autoimmune neuropathy, there may be variability in disease severity, and it does not fully replicate every aspect of human autoimmune neuropathies, particularly in terms of chronicity and long-term outcomes.
Published Data
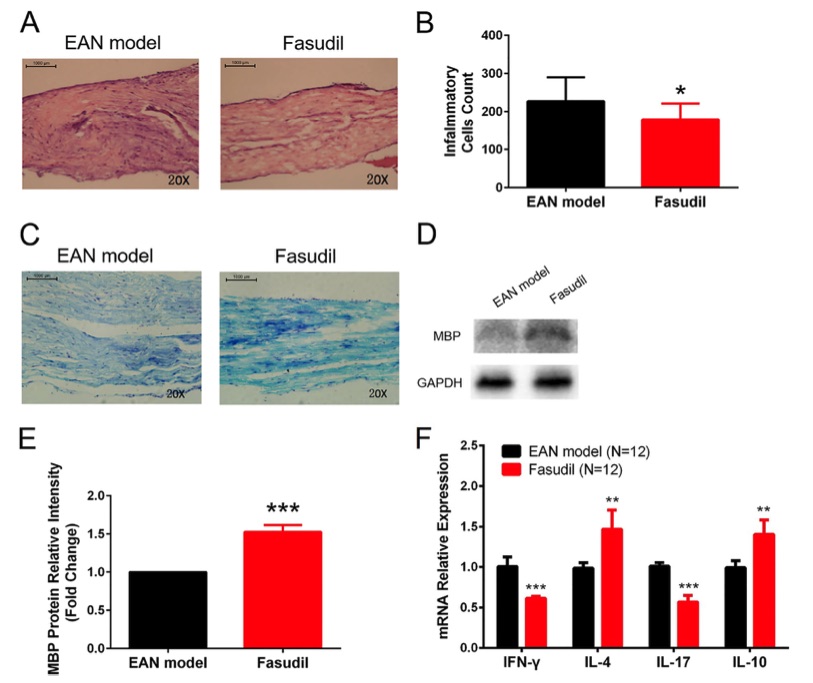 Fig. 1 Sciatic nerves inflammation and demyelination in experimental autoimmune neuritis (EAN) mice treated or not with fasudil.1
Fig. 1 Sciatic nerves inflammation and demyelination in experimental autoimmune neuritis (EAN) mice treated or not with fasudil.1
The therapeutic effect of fasudil in treating experimental autoimmune neuritis (EAN) was evaluated in this study. Histological analysis using HE staining revealed a significant reduction in inflammatory cell infiltration in the sciatic nerves of the fasudil-treated group compared to the EAN model group at day 28 (Figure 1A and B). Additionally, Luxol fast blue staining demonstrated a marked attenuation of demyelination in the sciatic nerves of the fasudil group (Figure 1C). Immunohistochemical analysis showed an increase in MBP expression in the sciatic nerves of the fasudil-treated animals (Figure 1D and E). Furthermore, qRT-PCR analysis revealed a significant decrease in the mRNA expressions of IFN-γ and IL-17, while the expressions of IL-4 and IL-10 were notably increased in the fasudil-treated group (Figure 1F). These findings suggest that fasudil effectively reduced both inflammation and demyelination in the sciatic nerves.
Reference
- Zhao, Yanyin et al. "Effect of fasudil on experimental autoimmune neuritis and its mechanisms of action." Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologica vol. 53,1 e8669. 20 Dec. 2019, doi:10.1590/1414-431X20198669. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1590/1414-431X20198669
For Research Use Only.
