Respiratory Disease Modeling & Pharmacodynamics Services
Introduction
Respiratory diseases, such as pneumonia, chronic obstructive pulmonary disease (COPD), rhinitis, asthma, cystic fibrosis (CF), pulmonary hypertension (PH), idiopathic pulmonary fibrosis (IPF), and tuberculosis (Tbc), contribute to 7% of global mortality annually. Primary pathogenic factors encompass allergen exposure, bacterial and viral pathogens, tobacco smoke, immunodeficiency, and genetic susceptibilities. Creative Biolabs has developed a variety of refined respiratory system animal models using diverse methodologies to accurately replicate the pathophysiological characteristics of respiratory diseases, which play a crucial role in advancing the R&D of pharmaceuticals. Furthermore, they provide verification methods for preclinical assessments of diverse therapeutic agents, including vaccines, small molecules, biological products, as well as single domain antibodies, gene therapies, and cell therapies.
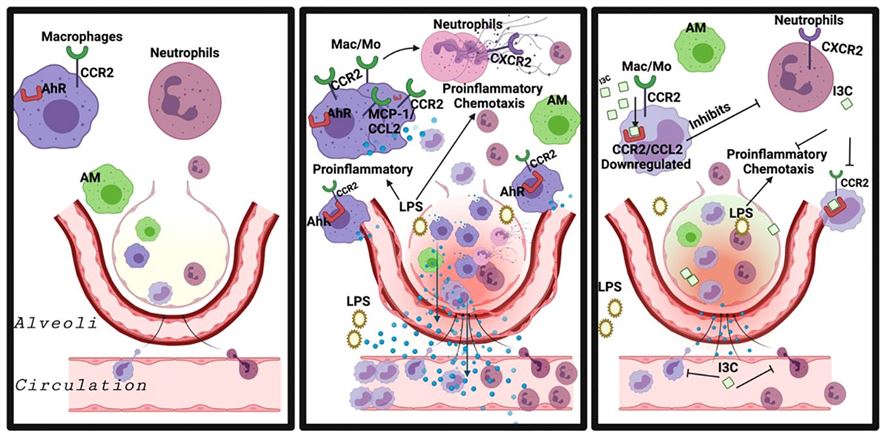 Fig.1 Exposure to LPS resulted in excessive neutrophil infiltration and significant lung tissue injury.1,4
Fig.1 Exposure to LPS resulted in excessive neutrophil infiltration and significant lung tissue injury.1,4
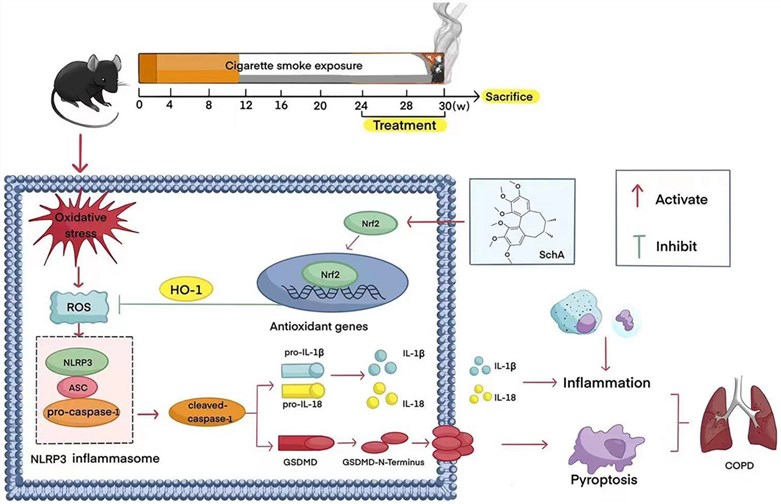 Fig.2 Schisandrin A modulates the Nrf2 signaling pathway to suppress NLRP3 inflammasome activation, thereby inhibiting cellular pyroptosis.2,4
Fig.2 Schisandrin A modulates the Nrf2 signaling pathway to suppress NLRP3 inflammasome activation, thereby inhibiting cellular pyroptosis.2,4
Available Respiratory System Disease Models
Creative Biolabs' existing well-characterized respiratory disease models for preclinical research include rodent, non-rodent, and non-human primates. Learn more about these models by clicking on the links below.
- Ovalbumin induced Asthma Model
- HDM induced Allergic Asthma Model
- MC903 & Ovalbumin induced Asthma Model
- OVA & HDM induced Asthma Model
- LPS & Ozone induced Asthma Model
- IL-17/TH17-Driven Asthma Model
- Poly(I:C) induced Acute Lung Injury Models
- Obesity-Related Asthma Model
- Bleomycin induced Pulmonary Fibrosis Model
- Silica induced Pulmonary Fibrosis Model
- Paraquat induced Pulmonary Fibrosis Model
- Asbestos induced Pulmonary Fibrosis Model
- TGF-beta Transgenic Mouse Pulmonary Fibrosis Model
- SP-C Mutant Mouse Pulmonary Fibrosis Model
- Telomerase-Deficient Mouse Pulmonary Fibrosis Model
- Adoptive Transfer of Fibrocytes Model
- IL-13/IL-17 Overexpression Induced Pulmonary Fibrosis Model
- LPS induced Pulmonary Neutrophilia Model
- LPS induced Acute Lung Injury Models
- Sephadex Ged induced Acute Lung Injury (ALI) Model
- High Oxygen Environment induced Acute/Chronic Lung Injury Model
- Papain induced Acute Lung Injury Models
- TNF-α and IL-17A induced Acute Lung Injury Model
- Immune Complex induced Acute Lung Injury Model
- A. Baumanii-infected Pneumonia Model
- Poly (I:C) induced Acute Lung Injury Model
- Cigarette Smoke induced COPD Model
- Cigarette Smoking & LPS induced COPD Mouse Model
- PPE induced Pulmonary Emphysema Models
- Human Neutrophil Elastase (HNE) Model
- LPS induced Pulmonary edema Model
- Unilateral Nephrectomy & DOCA induced Nephrogenic Pulmonary Edema Model
- Epinephrine/Norepinephrine induced Acute Cardiogenic Pulmonary Edema Model
- IL-13 induced Airway Mucus Hypersecretion Models
- Acetylcholine induced Airway Constriction Models
- Pseudomonas Aeruginosa induced Bronchiectasis Model
- Citric Acid induced Cough Model
- Citric Acid & ATP induced Cough Model
- Citric Acid & Histamine induced Cough Model
- Citric Acid &OVA induced Cough Model
- Citric Acid & Respitose induced Cough Model
- Capsaicine induced Cough Model
- Cinnamyl Aldehyde induced Cough Model
- Monocrotaline induced Pulmonary Hypertension Model
- Hypoxia induced Pulmonary Hypertension Model
- OVA induced Allergic Rhinitis Model
- HDM induced Allergic Rhinitis Model
- Capsaicin induced Neurogenic Rhinitis Model
- SiO2 induced Pulmonary Nodule Model
- Carbon nanoparticle induced sarcoidosis model
 Fig.3 Mammalian and invertebrate models available to study the chronic respiratory diseases.3,4
Fig.3 Mammalian and invertebrate models available to study the chronic respiratory diseases.3,4
Evaluation Platform
- Animals: Mouse, Rat, Hamster, Rabbit, Cat, Dog, NHPs.
-
Measurements
To thoroughly assess pharmacology and efficacy, ensuring scientific accuracy and data reliability, Creative Biolabs has established the following platforms:- Clinical Symptom and Sign Monitoring: This platform conducts evaluations of body weight changes, activity levels, survival status, respiratory function, pulmonary function tests, and gas exchange efficiency.
- Histopathological Analysis: Histopathological evaluation is conducted using anatomical techniques and various staining methods, including H&E staining, PAS staining, Masson's trichrome staining, immunohistochemistry (IHC), indirect immunofluorescence (IF), TUNEL staining, and the comet assay.
- Molecular Biology and Immunology Detection: This platform facilitates the detection of genes and immune cells through techniques such as RT-qPCR, digital PCR, in situ hybridization (ISH), flow cytometry, MSD, next-generation sequencing, and high-throughput screening.
- Imaging and In Vivo Dynamic Monitoring: The progression or scope of lesions is detected via small animal ultrasound, in vivo imaging, and Micro-CT.
Our advantages
- Professional technical capabilities: Our technical team comprises members with diverse and complementary professional backgrounds, with extensive knowledge and practical experience in areas such as respiratory pharmacology, anti-inflammatory and immunomodulatory drug development, and inhalable formulation development. Our team holds a leading position in respiratory disease drug development and preclinical evaluation, providing comprehensive and professional technical support for all projects.
- Diversity of detection platforms: We introduce internationally advanced testing instruments and offer advanced detection methods. This enables the comprehensive evaluation of inflammatory factors, histopathology, and immunology, thereby ensuring accuracy and professionalism in efficacy assessments.
- Project management capabilities: We have a project management team that works flexibly with project scientists and experimental technicians, adeptly handling challenges during project implementation, ensuring efficient and high-quality project completion.
- Service flexibility and customization: We offer tailored model services based on individual client R&D requirements and specific model characteristics, allowing for flexible adjustments during project execution to meet diverse customer needs.
- One-stop service: Our comprehensive services encompass all facets of preclinical research on respiratory diseases, including pharmacodynamic, toxicological, and pharmacokinetic research. This approach eliminates the need for multiple suppliers, saving time and effort while enhancing research and development efficiency.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q. How does Creative Biolabs select the appropriate respiratory disease animal model based on research objectives?
A: Choosing an effective model requires a comprehensive assessment of the particular disease, research aims, and translational needs. This approach is exemplified by the use of the ovalbumin-sensitized mouse model for asthma studies, the cigarette smoke-exposed rat model for COPD, and various models for acute lung injury in ARDS research. Such evaluations facilitate model applicability analyses and recommendations that are closely aligned with specific research objectives.
-
Q. How does Creative Biolabs ensure the stability and reproducibility of your models?
A: We strictly adhere to standardized procedures, meticulously controlling every stage, from the selection and husbandry of experimental animals, the source, dosage, and administration route of modeling compounds, to the definition of key model evaluation endpoints. Through extensive preliminary experiments and data analysis, we define the optimal parameter ranges for model induction, minimizing the impact of individual variations on model stability. Validated through long-term application, our models show minimal batch-to-batch variation and high data reliability. We've successfully provided preclinical Contract Research Organization (CRO) services to numerous pharmaceutical companies, contributing to the approval of multiple clinical trials.
-
Q. What are the core modeling technologies used in constructing respiratory disease models?
A: The core modeling techniques include chemical induction, immune stimulation, mechanical injury, and gene editing. We have mastered these techniques and continually optimize them to enhance model quality for pharmacology and pharmacodynamic studies.
-
Q. Does Creative Biolabs' team have the capability to perform complex experimental techniques?
A: Yes. Creative Biolabs' team exhibits extensive expertise in advanced experimental techniques, spanning several key areas. For example, molecular biology (e.g., bioinformatics analysis and proteomics), cell biology (e.g., cell culture, cell transfection, and cell functional assays), intricate surgical procedures (e.g., lobectomy and tracheal intubation), and cutting-edge imaging technologies.
Published Data
The effect of Indole-3-carbinol (I3C) in acute respiratory distress syndrome (ARDS) induced by LPS: Following 48-hour treatment with LPS or LPS + I3C, murine pulmonary immune cells analysis demonstrated that I3C directly suppressed the infiltration of both CCR2+ monocytes and CXCR2+ neutrophils within the lung.
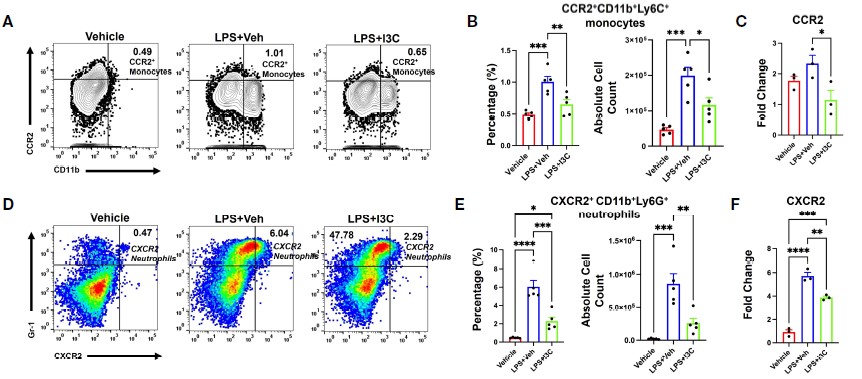 Fig.4 LPS exposure leads to an expansion of both CXCR2+ monocyte and CXCR2+ neutrophil populations, an effect that is significantly attenuated by I3C treatment.1,4
Fig.4 LPS exposure leads to an expansion of both CXCR2+ monocyte and CXCR2+ neutrophil populations, an effect that is significantly attenuated by I3C treatment.1,4
References
- Holloman, Bryan Latrell et al. "Indole-3-carbinol attenuates lipopolysaccharide induced acute respiratory distress syndrome through activation of AhR: role of CCR2+ monocyte activation and recruitment in the regulation of CXCR2+ neutrophils in the lungs." Frontiers in Immunology vol. 15 1330373. https://doi.org/10.3389/fimmu.2024.1330373
- Zeng, Jiamin et al. "Schisandrin A regulates the Nrf2 signaling pathway and inhibits NLRP3 inflammasome activation to interfere with pyroptosis in a mouse model of COPD." European Journal of Medical Research vol. 28,1 217. https://doi.org/10.1186/s40001-023-01190-8
- Fröhlich, Eleonore. "Animals in Respiratory Research." International Journal of Molecular Sciences vol. 25,5 2903. https://doi.org/10.3390/ijms25052903
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
