Non-Allergic Asthma Modeling & Pharmacodynamics Services
Introduction
Non-Allergic Asthma does not rely on IgE-mediated allergic reactions which constitutes a substantial proportion of adult asthma cases, particularly in elderly patients, and accounts for approximately 20%-30% of pediatric cases. Clinically, it encompasses phenotypes such as neutrophilic asthma, and obesity-related and virus-associated subtypes. The related models are primarily established through chemical induction, viral stimulation, or neurogenic stimulation. Creative Biolabs has successfully established non-allergic asthma animal models that are meticulously designed to align with various pathogenic mechanisms. These models are crucial for advancing preclinical drug research and development, providing essential insights into disease mechanisms, identifying novel therapeutic targets and biomarkers, aiding vaccine development, and assessing innovative therapies.
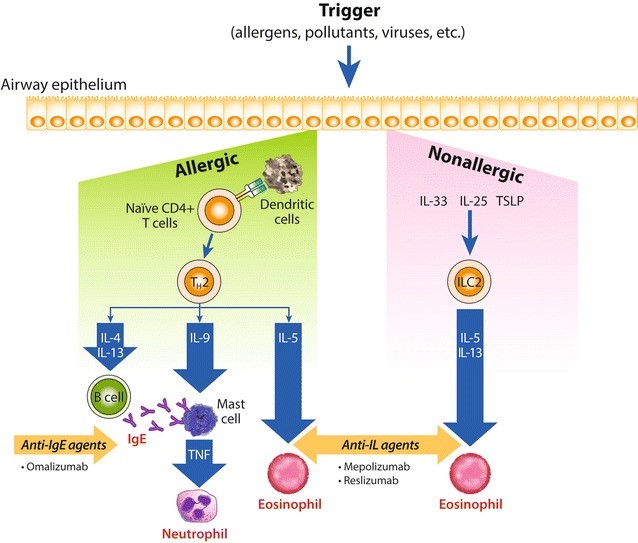 Fig.1 Allergic and non-allergic inflammatory asthma cascade.1,5
Fig.1 Allergic and non-allergic inflammatory asthma cascade.1,5
Available Non-Allergic Asthma Models
| Non-Allergic Asthma Models | Modeling and applications | Animal Species |
| LPS & Ozone-Induced Asthma Model | Environmental pollutant-induced asthma, suitable for environmental exposure mechanisms, antioxidant, anti-neutrophil extracellular traps (NETs) therapies, or biomarker development. | Mouse, Rat |
| IL-17/TH17-Driven Asthma Model | Severe, mixed, childhood, neutrophilic, steroid-resistant asthma, extensively used in Asthma Pathogenesis, Impact of Environmental Factors, Biologic therapy, such as Anti-IL-17/IL-23 biologics, JAK inhibition. | Mouse |
| Poly(I:C)-Induced Acute Lung Injury Models | Infection-triggered acute lung injury, crucial for viral ARDS pathogenesis and therapeutics, such as NLRP3-targeted drugs, anti-inflammatory drugs, immunomodulators, and vaccines | Mouse |
| Obesity-Related Asthma Model | Obesity-associated refractory asthma, excellent for new therapies targeting the metabolic-inflammation axis, such as the insulin sensitizer, GLP-1 receptor agonists, SGLT2 inhibitors, adiponectin agonists, and leptin antagonists | Mouse, Rat |
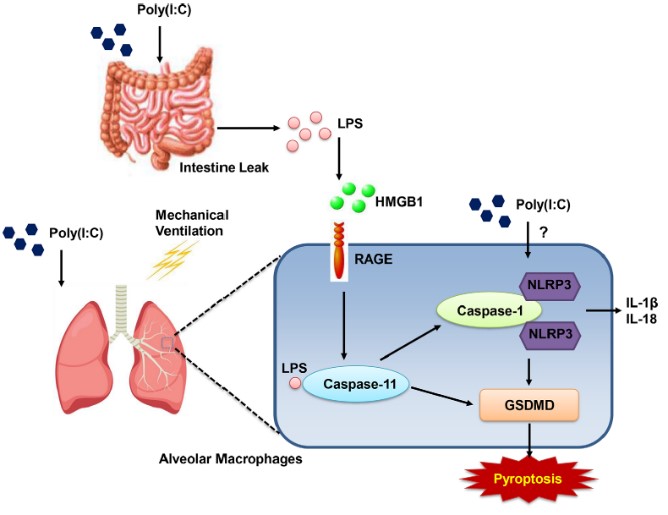 Fig.2 Combined injury induced by poly(I:C) and moderate tidal volume ventilation (MTV) led to increased gastrointestinal permeability, along with elevated endotoxin levels in both plasma and bronchoalveolar lavage fluid (BALF).2,5
Fig.2 Combined injury induced by poly(I:C) and moderate tidal volume ventilation (MTV) led to increased gastrointestinal permeability, along with elevated endotoxin levels in both plasma and bronchoalveolar lavage fluid (BALF).2,5
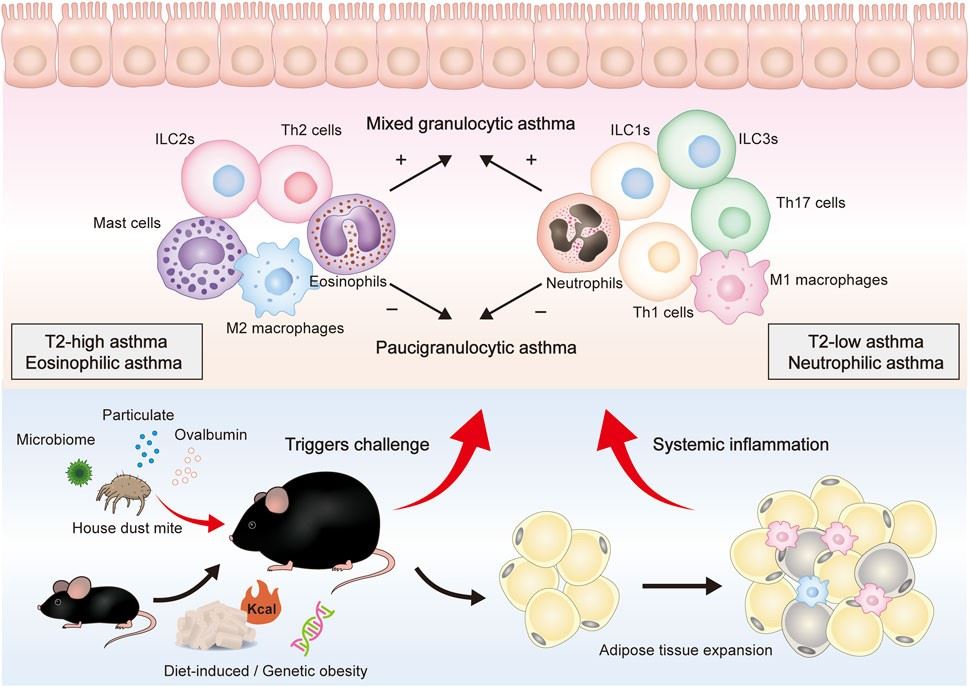 Fig.3 Overall presentation of obesity-related asthma development and the putative roles of different immune cells in the airway.3,5
Fig.3 Overall presentation of obesity-related asthma development and the putative roles of different immune cells in the airway.3,5
Evaluation Platform
We provide a multi-dimensional detection platform to ensure comprehensive and accurate results, as follows:
- Biochemical Detection: Quantitative analysis will be performed on inflammatory factors, enzyme activities, and metabolites in biological fluids and tissues, such as inflammatory enzymes, chemokines/cytokines, and markers of oxidative stress.
- Molecular Detection: A comprehensive assessment of gene transcription and protein abundance will be conducted to elucidate underlying molecular mechanisms.
- Cell Detection: This includes the assessment of parameters such as cell viability and specific cellular functions relevant to inflammation. Alternatively, primary cells, such as peripheral blood mononuclear cells (PBMCs), can be isolated and verified to have the cell functions to accurately simulate the in vivo inflammatory microenvironment.
- Histopathological Detection: The extent of tissue damage will be quantitatively assessed through morphological evaluation, employing methods such as semi-quantitative scoring and immunohistochemistry.
- Behavioral Detection: Asthma-related respiratory symptoms will be evaluated, including parameters such as airway function and airway hyperresponsiveness (AHR).
- Imaging Detection: The dynamic observation of lung structure and inflammatory distribution in vivo will be conducted using lung functional imaging, such as Micro-CT, nuclear Imaging, in vivo magnetic resonance microscopy (MRM), and real-time in vivo imaging.
Applications
- Novel Target Validation: Using specialized non-allergic asthma animal models to test and validate novel drug targets that address non-Th2 inflammatory pathways (e.g., neutrophilic or T1/T17 inflammation).
- Candidate Drug Screening and Evaluation: Evaluating the efficacy and safety of new chemical entities or biologics by testing their impact on key non-allergic asthma features.
- Disease Mechanism and Biomarker Research: Models are used to gain a deeper understanding of the specific triggers and molecular pathways involved in non-allergic asthma (e.g., the role of innate immune responses and environmental factors), also used to identify and validate novel biological markers (e.g., specific cytokines, inflammatory cells, or gene signatures) that are characteristic of non-allergic asthma.
Our advantages
- Non-allergenic Mechanism Specificity: We focus on developing asthma models that are not dependent on IgE mediation. These models address the market gap for non-Th2 type inflammation models, accurately simulating the pathological characteristics of non-allergenic asthma patients.
- Multi-dimensional Preclinical Evaluation Capability: Leveraging multi-dimensional detection platforms such as pulmonary function tests, inflammatory cell typing, imaging, and histopathological scoring systems, we comprehensively evaluate molecular mechanisms from multiple perspectives, and ensure a high degree of concordance between the model's inflammatory pathways and clinical etiologies. Our approach efficiently validates the efficacy of diverse drug types, such as inhaled formulations and small-molecule inhibitors, thereby accelerating drug discovery timelines.
- Advanced Facilities and Technological Platforms: We employ state-of-the-art instruments and advanced technological methods to detect model-related indicators. We're equipped with aerosol exposure systems, a FinePointe pulmonary function test system, and a flow cytometry analysis room. This setup enables full-process services, from model establishment to efficacy evaluation, ensuring accurate and scientifically sound results.
- Frontier Theories Integration: By integrating frontier theories such as the gut-lung axis and metabolism-immune interactions, we simulate complex clinical etiologies, thereby enhancing the translational value of our models.
- Quality Control Advantages: Internal quality control is maintained through SOP standardized management, which includes practices like preliminary dose-gradient experiments for model induction and data analysis. We meticulously track and provide feedback on experimental progress and data, ensuring the reproducibility of results (with a model success rate of 90%).
- Flexible Model Development Solutions: We have extensive modeling experience, allowing us to quickly establish new, customized models based on client specifications, including variations in inducing agents, animal species, specific genetic backgrounds of mice, or combined modeling, for comprehensive technical support throughout the entire process, from model generation to mechanistic analysis.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What are the core differences between non-allergic asthma models and allergic asthma models?
A: Non-allergenic asthma, such as obesity-related asthma models and virus-induced types, does not rely on IgE-mediated immune responses but triggers AHR and inflammation through other mechanisms, such as airway epithelium damage, innate immune activation, and mixed inflammation stimulation. In contrast, allergenic asthma depends on Th2-type immune responses induced by allergens, such as OVA and HDM.
-
Q: How can the pathological relevance of non-allergic asthma models be validated?
A: Validation indicators include pulmonary function tests (e.g., airway resistance and compliance), the proportion of non-Th2 type inflammatory cells (e.g., neutrophils and macrophages), cytokine profiles (e.g., IL-8, TNF-α increased), and histopathology without significant eosinophilic infiltration (e.g., proliferation of airway smooth muscle, high mucus secretion).
-
Q: How to ensure the success rate of non-allergic asthma modeling?
A: We are equipped with a comprehensive detection platform, and our experimental and animal housing areas are clearly separated. All detection equipment undergoes regular calibration, and we maintain complete maintenance records. Simultaneously, we strictly control critical modeling factors, such as Poly(I:C) dosage, administration route, and high-fat diet duration. We also implement rigorous Standard Operating Procedures (SOPs) for model construction, data acquisition, and animal welfare management. For instance, rapid assessment of inflammatory characteristics via BALF cell counts and lung tissue H&E staining within 48-72 hours post-induction helps to exclude unsuccessful model samples.
-
Q: What are the advantages of non-allergenic asthma models in drug development?
A: Non-allergic asthma models are primarily constructed by addressing key aspects of the disease not covered by traditional allergic models, offer several significant advantages in drug development: mimicking a broader patient population, elucidating non-th2 inflammatory mechanisms, screening drugs for specific inflammatory phenotypes, evaluating drug breadth and specificity, exploring mechanisms of steroid resistance and novel treatment strategies, validating the role of infections and environmental factors.
-
Q: Can the models be used to evaluate new drug delivery routes (such as inhalation formulations)?
A: Yes, the aerosol exposure system can simulate inhalation administration. After modeling, the model can be used to evaluate the drug's effect on reducing airway inflammation and AHR. Additionally, lung tissue drug distribution assessments (such as mass spectrometry imaging) can confirm targeting efficacy.
Published Data
The Diet-Induced Obesity (DIO) mouse model, which mimics human obesity-related pathological conditions, showed that the hypoglycemic drug Metformin has no effect on body weight and does not significantly reduce blood glucose or TNF-α mRNA levels, but significantly reduces serum insulin levels and AHR. This case demonstrates the capability of the obesity-related asthma model for PD studies.
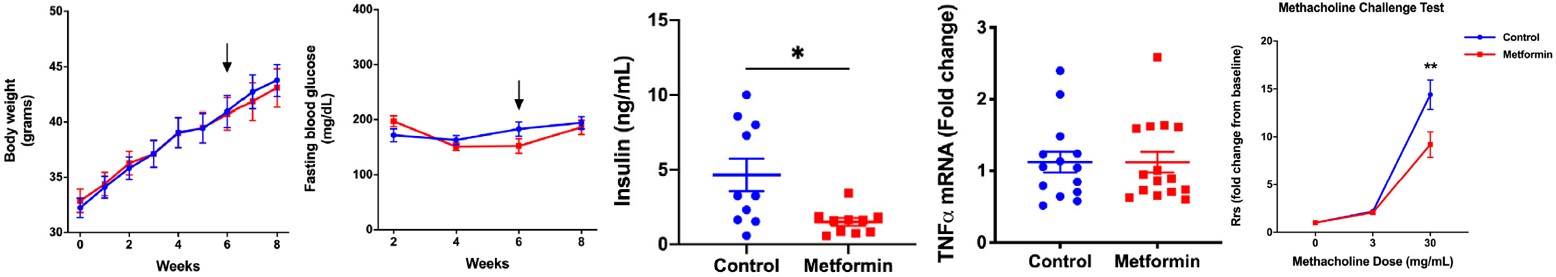 Fig.4 Metformin's impact on body weight, blood glucose, insulin, TNF-α mRNA, and AHR in high-fat diet-induced obese C57BL/6J mice.4
Fig.4 Metformin's impact on body weight, blood glucose, insulin, TNF-α mRNA, and AHR in high-fat diet-induced obese C57BL/6J mice.4
References
- Kim, Harold et al. "Asthma biomarkers in the age of biologics." Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology vol. 13 48. https://doi.org/10.1186/s13223-017-0219-4.
- Jin, Shuqing et al. "Mechanical Ventilation Exacerbates Poly (I:C) Induced Acute Lung Injury: Central Role for Caspase-11 and Gut-Lung Axis." Frontiers in immunology vol. 12 693874. https://doi.org/10.3389/fimmu.2021.693874.
- Kong, Jingwei et al. "Airway immune response in the mouse models of obesity-related asthma." Frontiers in physiology vol. 13 909209. https://doi.org/10.3389/fphys.2022.909209.
- Gu, Chenjuan et al. "Metformin Alleviates Airway Hyperresponsiveness in a Mouse Model of Diet-Induced Obesity." Frontiers in physiology vol. 13 883275. Distributed under Open Access license CC BY 4.0, with modification. https://doi.org/10.3389/fphys.2022.883275.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
