Atopic Dermatitis & Eczema Modeling & Pharmacodynamics Services
Creative Biolabs offers a variety of well-established and reliable models to evaluate the efficacy of drug candidates for atopic dermatitis and eczema, providing a comprehensive platform for preclinical research.
Introduction
Atopic dermatitis (AD) and eczema are chronic inflammatory skin disorders that often cause intense itching, redness, and irritation. These conditions are prevalent worldwide and can affect individuals of all ages, though AD is particularly common in children. Eczema refers to a group of conditions characterized by inflamed, irritated skin, with atopic dermatitis being the most common form. The exact cause of these conditions remains unclear but is believed to involve a combination of genetic predisposition, immune system dysfunction, and environmental factors such as allergens, irritants, and microbial infections. Both AD and eczema are associated with disruptions in the skin barrier, which allows irritants and allergens to penetrate the skin, leading to inflammation and allergic responses. These diseases are often chronic, with periods of flare-ups and remissions. The immune system, particularly T cells, plays a central role in driving inflammation and immune responses in the skin. Treatment strategies aim to manage symptoms, reduce inflammation, and restore the skin barrier. Common therapies include corticosteroids, calcineurin inhibitors, and newer biologics. However, the complexity and chronic nature of these diseases highlight the need for ongoing research to develop more effective and targeted treatments. Animal models of atopic dermatitis and eczema are invaluable for studying disease mechanisms and testing potential therapies.
Atopic Dermatitis and Eczema Models and Applications
Creative Biolabs offers a variety of well-established rodent models for atopic dermatitis (AD) and eczema. These models are carefully designed to replicate the inflammatory skin conditions seen in humans, including immune dysregulation and skin barrier dysfunction. They provide invaluable tools for evaluating the efficacy of therapeutic candidates in preclinical research. Our expert team of scientists will collaborate with you throughout your project, from designing the experiment to interpreting the data, ensuring high-quality and reliable results. To learn more about the atopic dermatitis and eczema models available for preclinical research, please explore the links below:
| Models | Simulates | Evaluates Drugs | Animal species |
| Dinitrofluorobenzene (DNFB) induced Atopic Dermatitis Model | Allergic contact dermatitis and atopic dermatitis | Topical corticosteroids, Calcineurin inhibitors, Antihistamines, Immunomodulators, Anti-inflammatory agents | Mouse |
| House Dust Mite (HDM) induced Atopic Dermatitis Model | House dust mite induced atopic dermatitis | Biologics (e.g., monoclonal antibodies), Topical corticosteroids, Immunotherapy, Antihistamines, Barrier repair agents | Rat, Mouse |
| Oxazolone (OXZ) induced Atopic Dermatitis Model | Chemical induced atopic dermatitis | Topical treatments, Immunosuppressive drugs, Antihistamines, Anti-inflammatory drugs, Biologics | Mouse |
| Ovalbumin (OVA) induced Atopic Dermatitis Model | Allergic asthma and atopic dermatitis | Immunotherapy, Corticosteroids, JAK inhibitors, Barrier repair agents, Antihistamines | Mouse, Rabbit |
| Calcipotriol (MC903) induced Atopic Dermatitis Model | Psoriasis-like atopic dermatitis | Topical calcineurin inhibitors, Vitamin D analogs, Anti-inflammatory drugs, Immunosuppressive agents | Mouse |
| Fluorescein Isothiocyanate (FITC) induced Atopic Dermatitis Model | Chemical induced allergic contact dermatitis | Antihistamines, Topical corticosteroids, Anti-inflammatory agents, Immunomodulators | Mouse |
| Ovalbumin (OVA) & Calcipotriol (MC903) induced Atopic Dermatitis Model | Combined model of allergic and inflammatory dermatitis | Topical corticosteroids, JAK inhibitors, Antihistamines, Biologics, and immunosuppressants | Mouse, Dog, Mini pig |
Evaluation Platform
- Animals: Mouse, Rat, Rabbit, Dog, Mini pig.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in rodent models of atopic dermatitis and eczema, utilizing advanced technologies, including but not limited to:- General observations: Skin redness, thickness, lesion severity, and behavioral signs such as scratching frequency.
- Histological analysis: Skin tissue samples examined for epidermal thickness, immune cell infiltration (e.g., T-cells, eosinophils), and tissue damage.
- Cytokine profiling (e.g., ELISA): Expression levels of key inflammatory mediators such as IL-4, IL-13, TNF-α, and IL-22.
- Serum biomarkers: Total IgE levels, eosinophil count, and other immune markers related to allergic responses.
- Gene/protein expression profiling: Using RT-qPCR and Western blot to analyze skin-specific proteins like filaggrin, as well as cytokines involved in inflammation and barrier function.
- Skin barrier integrity assays: Measurement of transepidermal water loss (TEWL) to assess skin permeability and barrier function.
In addition to these standard measurements, our team can assist in developing customized experimental protocols tailored to your specific research needs, ensuring that your project benefits from a thorough and targeted approach.
Our advantages
- Comprehensive platform: Access to a variety of validated models for both allergic and irritant induced eczema and AD.
- Customizable protocols: Tailored experimental designs to meet your specific research requirements.
- Expert scientific support: Our team provides ongoing guidance and data analysis assistance throughout the research process.
- Advanced measurement techniques: A wide range of analytical tools, including histology, cytokine profiling, and gene expression analysis, for in-depth insights.
- Reliable and reproducible results: Proven, reliable models that provide consistent data for preclinical evaluation.
- Broad therapeutic scope: Evaluate a wide range of therapeutic approaches, from topical treatments to systemic biologics and immune modulators.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What types of models do you offer for AD and eczema?
We offer both chemical induced models (e.g., DNFB) and genetic models, such as the NC/Nga mouse model, to simulate atopic dermatitis and eczema.
-
2. Can the models be customized to test specific drugs?
Yes, we provide customizable experimental designs to assess various drug classes, including corticosteroids, immunomodulators, and new biologics.
-
3. What biomarkers do you measure in your models?
We measure a range of biomarkers, including cytokines (e.g., IL-4, IL-13), IgE levels, and markers of skin barrier function such as transepidermal water loss (TEWL).
-
4. How long does it take to observe significant effects in these models?
The duration of the experiment depends on the specific model and drug being tested, but typical studies last between 2-4 weeks.
-
5. Can you assess long-term therapeutic effects in your models?
Yes, our models allow for both short-term and chronic studies to evaluate the long-term effects of treatments on disease severity and recurrence.
Published Data
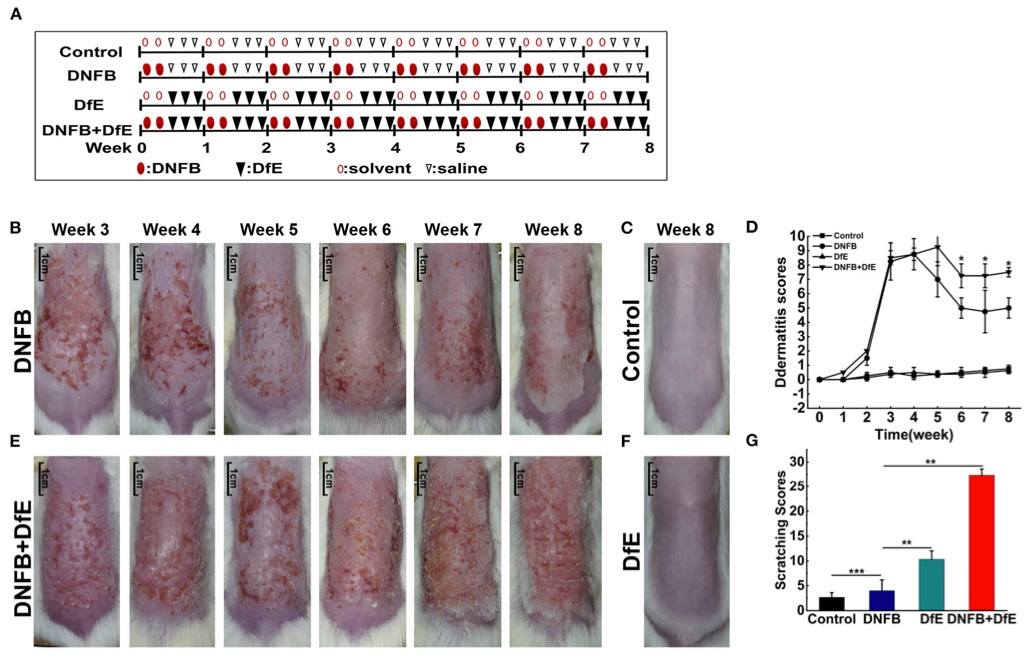 Fig. 1 Atopic dermatitis (AD)-like clinical features were induced by alternate application of dinitrofluorobenzene (DNFB) and an extract of Dermatophagoides farinae (DfE) in BALB/c mice. 1
Fig. 1 Atopic dermatitis (AD)-like clinical features were induced by alternate application of dinitrofluorobenzene (DNFB) and an extract of Dermatophagoides farinae (DfE) in BALB/c mice. 1
Atopic dermatitis (AD)-like clinical features were induced in BALB/c mice through the alternate application of dinitrofluorobenzene (DNFB) and an extract of Dermatophagoides farinae (DfE) (Figure 1A). The experimental design aimed to compare the effects of different exogenous agents on skin lesions in mice. Changes in the severity of skin lesions were recorded (Figures 1B–F). After 3 weeks of induction, the mice in the DNFB and DNFB+DfE groups developed severe erythema, erosion, scarring, and excoriation on the entire dorsal skin. However, clinical symptoms showed signs of relief, and dermatitis scores plateaued over time. Notably, skin lesions were significantly more severe in the DNFB+DfE group compared to the DNFB group. Chronic lesions, characterized by scaly patches, plaques with excoriation, and lichenification, were observed exclusively in the DNFB+DfE group. No skin lesions were seen in the DfE-only and control groups. AD-like lesions in BALB/c mice appeared only after alternate exposure to DNFB and DfE. After 7 weeks of stimulation, the scratching behavior was recorded over a 10-minute period, and cumulative scores were obtained (Figure 1G). The frequency of scratching was significantly higher in the DNFB+DfE and DfE groups compared to the other groups, with the DNFB+DfE group showing the highest frequency, though no statistical differences were found between these two groups. Scratching behavior was also observed in the DNFB and control groups, but with much lower frequency, and the DNFB group exhibited slightly higher frequency than the control group. These results indicate that scratching behavior was primarily induced by DfE and exacerbated by DNFB.
Reference
- Feng, Shujing et al. "An Atopic Dermatitis-Like Mouse Model by Alternate Epicutaneous Application of Dinitrofluorobenzene and an Extract of Dermatophagoides Farinae." Frontiers in Medicine vol. 9 843230. 15 Jun. 2022. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fmed.2022.843230
For Research Use Only.
