Genetic & Transgenic Pulmonary Fibrosis Modeling & Pharmacodynamics Services
Introduction
A subset of idiopathic pulmonary fibrosis (IPF) patients presents with a family history; its molecular pathogenesis may involve telomere pathway mutations, surfactant abnormalities, and epigenetic dysregulation. It may be associated with the interplay of genetics, specific populations, smoking, and occupational exposures. Key genes include TERT/TERC, MUC5B promoter polymorphism, SFTPC/SFTPA2, and TINF2. Creative Biolabs offers Genetic & Transgenic Pulmonary Fibrosis Models, unlike chemically induced models, these models more accurately recapitulate human genetic susceptibility, making them indispensable for advancing IPF research. They enable precise dissection of gene function, identification of therapeutic targets, and preclinical testing of mechanism-based interventions, thereby bridging the gap between bench and bedside. Combining the advantages of different models allows for a more comprehensive simulation of IPF pathological features, driving progress in research on IPF pathogenesis and clinical treatment.
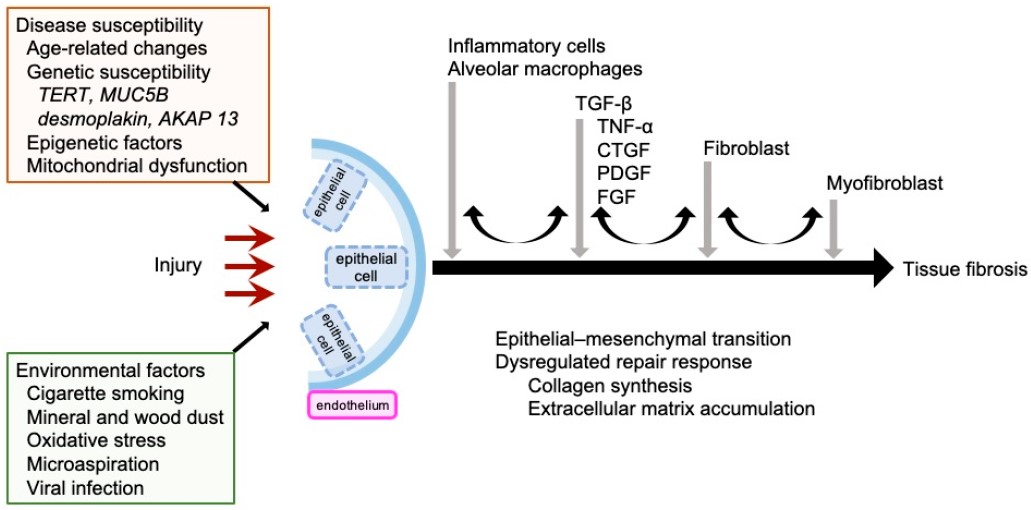 Fig.1 Mechanisms of IPF pathogenesis.1,3
Fig.1 Mechanisms of IPF pathogenesis.1,3
Available Genetic & Transgenic Pulmonary Fibrosis Models
| Pulmonary Fibrosis Models | Clinical Relevance | Primary Research Applications | Animal Species |
| TGF-beta Transgenic Mouse Pulmonary Fibrosis Model | Human IPF, Sporadic IPF. | Anti-fibrotic agents that target the TGF-β signaling cascade (e.g., TGF-β inhibitors, Smad inhibitors), and gene therapy approaches. | Mouse |
| SP-C Mutant Mouse Pulmonary Fibrosis Model | Simulates PF arising from alveolar epithelial cell dysfunction, familial IPF, and pulmonary alveolar proteinosis. | Agents targeting ER Stress pathways (e.g., ER stress inhibitors, ATF6 modulators), and molecular chaperone regulators designed for epithelial cell protection. | Mouse |
| Telomerase-Deficient Mouse Pulmonary Fibrosis Model | Telomere shortening and cellular senescence, heritable and sporadic IPF. | Anti-aging therapies (e.g., senolytics), agents targeting senescence-related fibrosis, and telomere-targeted therapies aimed at lengthening telomeres or stabilizing T cells. | Mouse |
Evaluation Platform
Our professional evaluation platform, refined over years of experience, ensures service reliability.
- Histopathology: We employ histological techniques, including H&E, Masson’s trichrome, and Sirius red staining, to assess structural damage and evaluate the effectiveness of therapeutic interventions.
- Molecular Profiling: We utilize diverse molecular techniques, including qPCR, RNA-seq, and NanoString, to identify dysregulated pathways and potential biomarker candidates.
- Protein Analysis: We employ protein analysis techniques, such as Western blot, ELISA, and Luminex multiplex assays, to verify alterations at the protein level and to elucidate cytokine networks.
- Cellular Phenotyping: We utilize advanced methodologies, including flow cytometry and immunofluorescence, to evaluate immune cell infiltration and responses specific to particular cell types.
- Functional Assays: We apply Functional assay techniques, such as hydroxyproline assays and lung compliance measurements, to correlate biochemical alterations with biomechanical changes.
Simultaneously, we integrate general clinical observations, organ indices, small animal in vivo imaging, and bioluminescence techniques for a comprehensive evaluation of our models and drug efficacy.
Applications
- Disease Modeling: These models simulate human genetic risks and, crucially, exhibit chronic, progressive fibrosis. This feature moves beyond temporary chemical injury to provide a truly translational platform for advanced disease study.
- Mechanistic Studies: Used to pinpoint core pathogenic drivers and molecular mechanisms.
- Drug Screening: Enables high-precision screening of compounds that target specific genetic or molecular defects. Ideal for testing inhibitors against core pathways and for evaluating novel anti-senescence and drug repurposing strategies.
- Drug R&D: The preferred platform for evaluating therapeutic efficacy against established, advanced fibrosis, the most clinically relevant stage. Essential for validating novel biomarkers and accelerating the development of advanced modalities like gene therapy and specialized cell therapies.
Our advantages
- Diverse Model Portfolio: We offer a broad selection of transgenic, knockout, and induced models, each precisely tailored to specific research goals.
- Multi-Omics Integration: We combine histopathology, transcriptomics, and proteomics to provide comprehensive insights.
- High-Resolution Imaging: Utilize Micro-CT and second-harmonic generation (SHG) microscopy for detailed 3D visualization of fibrosis.
- Disease-Relevant Endpoints: Our endpoints, such as lung function and survival, are aligned with clinical outcomes to ensure high translational relevance.
- Regulatory Compliance: All protocols adhere to GLP standards, ensuring preclinical data suitable for IND submissions.
- One-Stop Service: We offer a complete service from model design to data analysis, thereby reducing project fragmentation and streamlining your research.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What genetic backgrounds are available for transgenic models?
A: Standard backgrounds include C57BL/6J, BALB/c, and FVB/N. Custom backcrossing to other strains, such as NOD-SCID, is available upon request.
-
Q: Can models be customized to study gene-environment interactions?
A: Yes. We offer combinatorial models, such as telomerase-Deficient + repetitive bleomycin challenge, to simulate gene-environment interplay.
-
Q: What is the success rate for model generation?
A: >90% success rate guaranteed via optimized genotyping protocols and backup breeding strategies.
-
Q: How long does it take to establish a novel transgenic line?
A: Typically 12–16 weeks, depending on gene complexity and validation requirements. For instance, CRISPR knock-in/knockout models take 8–12 weeks (from zygote injection to F0 validation). Conditional models require 12–16 weeks (including LoxP allele breeding).
-
Q: Are your fibrosis scoring methods validated against clinical data?
A: Yes. Our histopathology metrics, such as the Ashcroft score, are cross-validated with human biopsy datasets.
-
Q: Do you support dose-ranging studies for therapeutics?
A: Certainly. We design multi-arm studies with pharmacokinetic/pharmacodynamic (PK/PD) profiling.
-
Q: How do you ensure model phenotype stability?
A: All models undergo quarterly phenotype revalidation, such as histology Lung function, and are maintained via heterozygous breeding to prevent genetic drift.
-
Q: Can you generate models with patient-derived mutations?
A: Absolutely. We accept patient genomic data to engineer custom mutations, such as point mutations or deletions, via CRISPR-HDR.
-
Q: What is the minimum cohort size for experiments?
A: Typically 6–8 mice/group for statistical power, adjustments based on endpoint variability, such as Lung function requires larger cohorts.
Published Data
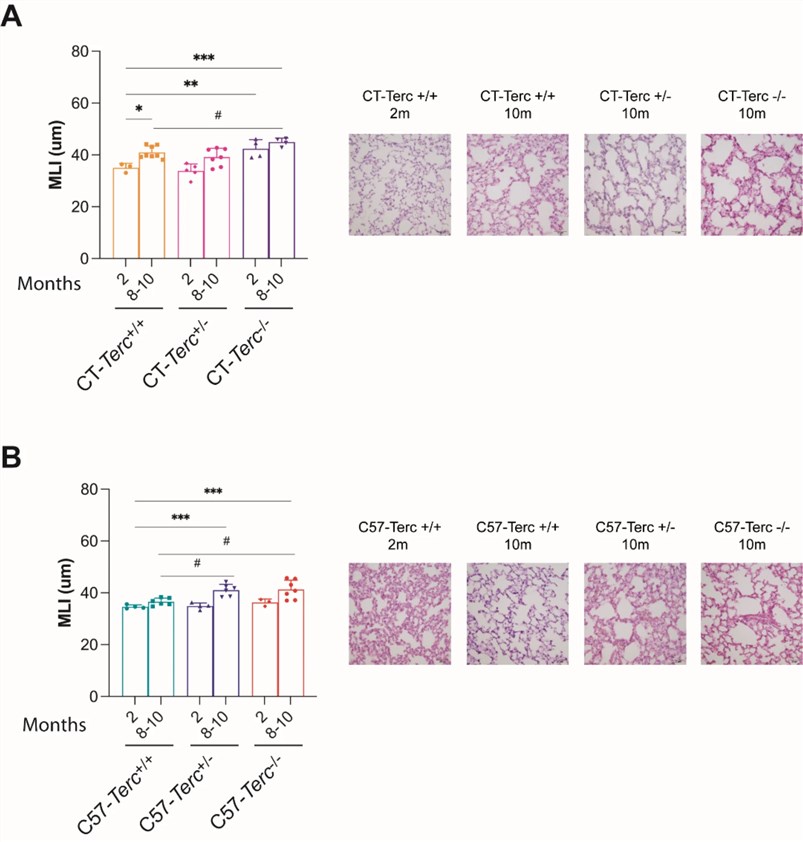 Fig.2 Analysis on lung parenchyma structure in Terc mutant mice.2,3
Fig.2 Analysis on lung parenchyma structure in Terc mutant mice.2,3
Terc mutant mice (particularly Terc-/-) exhibit an increase in the Mean Linear Intercept (MLI) at 8-10 months of age, indicating alveolar space enlargement and tissue architectural destruction. This reflects premature alveolar epithelial senescence, which is similar to the abnormal pulmonary structure observed in human TBDs, thus confirming the potential of the telomerase-deficient mouse pulmonary fibrosis model for pulmonary fibrosis R&D.
References
- Inui, Naoki et al. "Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to TGF-β and the Ubiquitin-Proteasome Pathway." International Journal of Molecular Sciences vol. 22,11 6107. https://doi.org/10.3390/ijms22116107
- Guerrero-López, Rosa et al. "Premature ageing of lung alveoli and bone marrow cells from Terc deficient mice with different telomere lengths." Scientific Reports vol. 15,1 6102. https://doi.org/10.1038/s41598-025-90246-2
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
