Experimental Sjögren's Syndrome (ESS) Modeling & Pharmacodynamics Services
Creative Biolabs offers a variety of well-established, optimized ESS models for drug efficacy evaluation, which can be tailored to specific research needs. Our models are ideal for testing the effectiveness of therapies aimed at controlling immune responses, alleviating glandular dysfunction, and improving patient outcomes.
Introduction
Experimental Sjögren's Syndrome (ESS) is a chronic autoimmune disease primarily affecting the exocrine glands, leading to reduced saliva and tear production, resulting in dry mouth (xerostomia) and dry eyes (keratoconjunctivitis sicca). The disease is characterized by the infiltration of immune cells, particularly T lymphocytes, into these glands, leading to inflammation, glandular atrophy, and dysfunction. In addition to the hallmark symptoms, ESS can cause systemic complications, including fatigue, joint pain, and involvement of other organs such as the kidneys, lungs, and liver. ESS is a valuable preclinical model for studying the pathophysiology of autoimmune diseases and for testing potential treatments. It mimics key features of human Sjögren's syndrome, including immune dysregulation, glandular damage, and systemic inflammation. The model can be induced through various methods, including the administration of autoantibodies or the use of genetically modified animals. These models provide insights into disease mechanisms, therapeutic strategies, and drug efficacy.
Experimental Sjögren's syndrome (ESS) Model and Applications
Creative Biolabs offers a variety of well-established rodent models for Experimental Sjögren's Syndrome (ESS). These models are carefully designed to replicate the key features of human ESS, including immune-mediated damage to the exocrine glands, dry mouth, dry eyes, and systemic inflammation. The models provide a valuable tool for testing new therapeutic strategies targeting the underlying autoimmune responses and glandular dysfunction associated with the disease. Our team of expert scientists will work closely with you throughout every stage of your research, from experimental design to data analysis, ensuring reliable, high-quality results. These models can be utilized to evaluate a range of therapeutic agents aimed at modulating immune responses, improving glandular function, and alleviating the systemic symptoms of ESS. For further information on how our ESS models can support your research needs, explore the following links below:
| Sjögren's Syndrome Model | Simulates | Evaluates Drugs | Animal species |
| SG Protein induced Sjögren's Syndrome Model | Autoimmune inflammation targeting salivary and lacrimal glands, resulting in dry mouth and dry eyes. | Immunosuppressive agents, corticosteroids, biologics targeting inflammatory cytokines (e.g., TNF-α inhibitors, rituximab), and drugs aiming to restore glandular function or alleviate dry eye symptoms. | Mouse |
Evaluation Platform
- Animals: Mouse.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in the Experimental Sjögren's Syndrome model, utilizing advanced technologies, including but not limited to:- General Observations: body weight, salivary gland function, lacrimal secretion, dry mouth, and eye scoring.
- Histological Analysis: Immune cell infiltration in salivary glands, lacrimal glands, and other affected tissues.
- Cytokine Profiling: Levels of pro-inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β) in serum and tissue samples.
- Immunohistochemistry: Detection of autoantibodies and immune markers (e.g., CD4+ T-cells, B-cells).
- Salivary Flow Test: Measurement of unstimulated and stimulated salivary flow to assess glandular dysfunction.
- Gene/Protein Expression Profiling: RT-qPCR and Western blot to assess molecular pathways involved in the disease.
In addition to established ESS models, we can help design customized models based on your research needs. Our team supports experimental design, data analysis, and model selection to ensure the most effective approach for your study.
Our advantages
- Customized Approach: Tailor the model to the specific stage of the disease for more accurate results.
- Comprehensive Evaluation: Wide range of measurements, from histological analysis to cytokine profiling, offering a holistic view of drug efficacy.
- Expert Support: Our scientific team guides you through model selection, experimental design, and data analysis to optimize your research outcomes.
- Wide Application: Applicable for testing various drug types, including biologics, small molecules, and immunotherapies.
- Proven Success: We have a track record of successful preclinical studies in ESS that contribute to the development of new therapeutic agents.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What are the key features of this model?
The ESS model replicates autoimmune symptoms, including dry mouth and eyes, salivary gland dysfunction, and systemic inflammation. It's useful for evaluating both local and systemic treatments.
-
2. How long does it take to observe results?
Typically, results can be observed within a few weeks, depending on the intervention. Long-term studies may be required for chronic treatment effects.
-
3. Can the model be used for drug safety testing?
Yes, this model is ideal for preclinical safety assessments of drugs targeting autoimmune diseases, including toxicity and long-term side effects.
-
4. What types of therapeutic agents can be tested?
Both small molecules and biologics, including monoclonal antibodies, cytokine inhibitors, and immunosuppressants, can be tested in this model.
Published Data
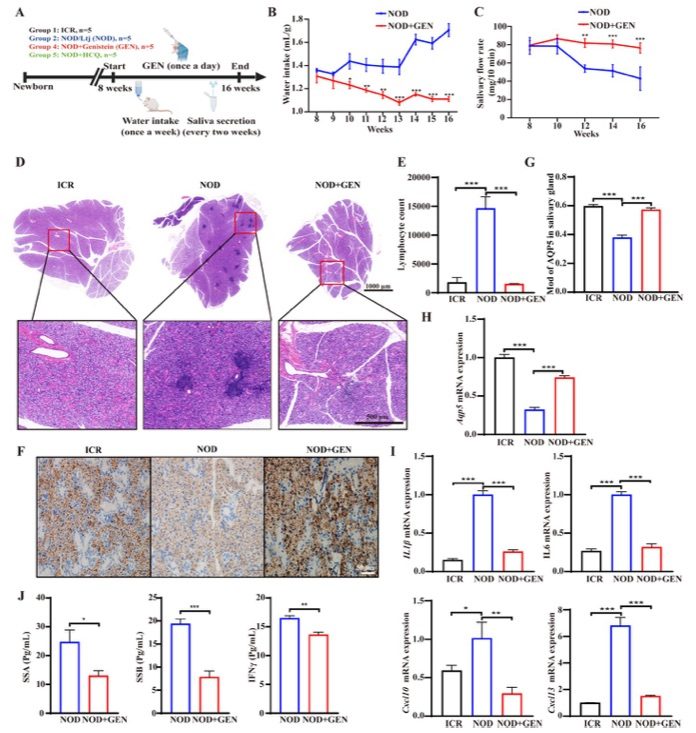 Fig. 1 Genistein reduced Sjögren's syndrome-related symptoms in NOD/LtJ mice.1
Fig. 1 Genistein reduced Sjögren's syndrome-related symptoms in NOD/LtJ mice.1
The effects of genistein on Sjögren's syndrome (SS) were assessed in vivo using NOD/LtJ mice. In untreated NOD/LtJ mice, an increase in water intake was observed, while genistein treatment significantly reduced water consumption (Fig. 1B). Furthermore, genistein increased the saliva flow rate in these mice (Fig. 1C), suggesting a reversal of salivary gland dysfunction and improvement of thirst symptoms. Focal lymphocytic sialadenitis (FLS) is a hallmark histopathological feature of SS. H&E staining of untreated NOD/LtJ mice revealed acinar atrophy, chronic inflammation, and an increased presence of FLS around perivascular and periductal regions of the submandibular glands. Genistein treatment improved these histopathological abnormalities (Fig. 1D) and reduced lymphocytic infiltration in the submandibular glands (Fig. 1E). Additionally, the expression of AQP5, a key protein involved in salivary gland water secretion, was analyzed. Immunohistochemistry (IHC) and RT-qPCR results showed that genistein significantly upregulated AQP5 expression in the submandibular glands of NOD/LtJ mice, approaching the levels found in ICR mice (Fig. 1F–H). Moreover, RT-qPCR analysis revealed that genistein downregulated the expression of inflammation-related genes, including Il1β, Il6, Cxcl10, and Cxcl13 (Fig. 1I).
Reference
- Mao, Tianjiao et al. "Salivary gland protective and antiinflammatory effects of genistein in Sjögren's syndrome by inhibiting Xist/ACSL4-mediated ferroptosis following binding to estrogen receptor-alpha." Cellular & Molecular Biology Letters vol. 29,1 147. 2 Dec. 2024. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1186/s11658-024-00667-6
For Research Use Only.
