Non-Allergic Rhinitis Modeling & Pharmacodynamics Services
Introduction
Non-allergic rhinitis (NAR) is a common condition defined as chronic nasal mucosal inflammation without clinical signs of intranasal infection or systemic allergic inflammation. It is a heterogeneous nasal disorder with a relatively high global incidence. It significantly impacts quality of life, leading to upper respiratory symptoms, sleep and psychological disturbances, and affecting work productivity and academic performance. Compared to patients with allergic rhinitis, those with non-allergic rhinitis often experience more pronounced and prolonged nasal congestion and rhinorrhea. While non-allergic rhinitis lacks a specific immunopathological background, its mechanisms involve epithelial changes or damage and neurokinin release, which may play a role in its pathogenesis.
 Fig.1 Classification and characteristics of NAR.
Fig.1 Classification and characteristics of NAR.
Creative Biolabs has developed various models to simulate the symptoms of non-IgE-mediated rhinitis. These models are used to explore existing treatment approaches, evaluate various crucial pathways in disease progression, and provide insights into the hyperresponsive mechanisms of non-allergic rhinitis.
Available Non-Allergic Rhinitis Model
| Non-Allergic Rhinitis Model | Clinical Relevance | Primary Research Applications | Animal Species |
| Capsaicin induced Neurogenic Rhinitis Model | NAR (Idiopathic/Vasomotor Rhinitis). Simulates nerve-driven symptoms (e.g., inflammation and sensory nerve activation). | TRPV1 Receptor Antagonists (to block sensory nerve activation), Neuropeptide Release Inhibitors (e.g., targeting Substance P), and Neuro-modulating agents used for chronic rhinitis. | Rat, Mouse, Guinea Pig |
Evaluation Platform
With years of accumulated experience, our leading technical platforms ensure the highest quality services and drive superior pharmacodynamic outcomes for your research.
- Macroscopic Phenotype and Behavioral Assessment: By observing nasal symptoms, behavioral changes, and responses to environmental stimuli, this assessment aims to ensure the animal model's presentation aligns with the symptoms of human non-allergic rhinitis.
- Nasal Mucosa Histopathology Detection: Analysis of the nasal mucosal structure and inflammatory status is performed to accurately differentiate between the various subtypes of non-allergic rhinitis based on underlying tissue pathology.
- Molecular and Biochemical Detection: Measuring inflammatory factors, chemokines, neurotransmitters, and vasoactive substances is essential to elucidate the underlying molecular pathogenesis and signaling pathways driving non-allergic rhinitis.
- Nasal Mucosa Functional Detection: Assessment of the barrier function, glandular secretory function, and vasomotor function of the nasal mucosa provides indicators that directly reflect the current pathological state and physiological impairment of the nose.
- Targeted Detection for Special Subtype Models: Specific measurements, such as nasal mucosal thickness or blood vessel wall thickening, are used to compare and contrast different rhinitis types, ensuring the model accurately represents a particular non-allergic subtype.
Applications
- Disease Simulation: Direct nerve stimulation by capsaicin accurately replicates the pathological states of neuronal hyperreflexia and glandular hypersecretion seen in these conditions.
- Mechanistic Analysis: The core application is to dissect the neurogenic inflammation pathway, investigating how TRPV1 activation leads to the release of neuropeptides and subsequent inflammation. It is also used for the functional and classification study of sensory neuron subtypes.
- New Drug Development: This model is a key platform for screening and validating TRPV1 antagonists and neuropeptide pathway modulators. It is also used to assess the desensitization effects of high-dose capsaicin on sensory nerves for developing novel neuro-modulatory therapies.
Our advantages
- Professionalism and Diversity in Model Construction: Our models are rigorously validated and can simulate the complex pathological mechanisms of different non-allergic rhinitis subtypes.
- In-depth Understanding of Pathological Mechanisms and Technical Translation Capabilities: By studying the mechanisms of non-allergic rhinitis involving multi-system interactions, such as nervous, vascular, and mucosal, we design targeted detection indicators based on different subtypes, enhancing the clinical translational value of our models.
- Customized Services and Flexible Project Design: We develop novel models and provide exclusive detection plans tailored to clients' specific mechanistic and subtype requirements.
- Data Reliability and Accumulated Experience: Through standardized operating procedures and extensive experience in constructing different species models, we can quickly resolve issues related to model construction and the selection of detection indicators.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What types of non-allergic rhinitis (NAR) animal models can your company construct?
A: We offer comprehensive testing across all mainstream Non-Allergic Rhinitis (NAR) models, including Neurogenic Inflammation (using TRPV1 agonists like capsaicin), Environmental Stimulus (ozone/SO2/cold air), Cholinergic Hyperactivity (carbachol-induced rhinorrhea), and Post-Infection models, with the capability to customize special subtypes.
-
Q: How do you ensure the clinical relevance of your models?
A: We analyze the clinical phenotype of the models using various indicators, including sneezing or nose-rubbing behavior, nasal resistance measurement, histological validation, and qPCR analysis of mucin genes.
-
Q: Can you provide humanized models?
A: Yes, we offer humanized approaches through transplant models, where human nasal mucosal tissue is transplanted into SCID mice, and transgenic models, specifically hTRPV1 overexpression mice (which require additional gene modification time).
-
Q: Besides non-allergic rhinitis model services, what other services do you offer?
A: We also provide other respiratory system model services, pharmacological and efficacy studies for other systemic diseases, and one-stop preclinical services, including toxicology.
Published Data
Intranasal stimulation with formaldehyde or capsaicin simultaneously induces local nasal mucosal inflammation and intracranial dural neurogenic inflammation. The significant correlation between their intensities directly supports the core hypothesis linking paranasal sinus inflammation, trigeminovascular system activation, and dural inflammation, providing experimental mechanistic backing for the clinical association between paranasal sinus diseases and migraine.
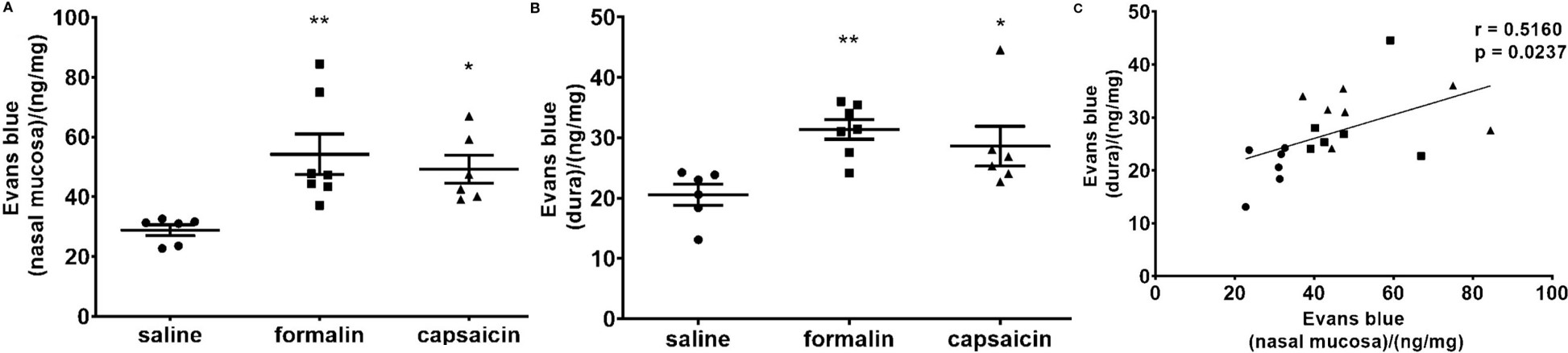 Fig.2 The inflammation was quantified by spectrophotometric measurement of formamide extracts of Evans blue dye (ng of the dye per mg of tissue in the nasal mucosa (A) and dura mater (B). (C) Individual animal values are plotted showing extravasation in the nasal mucosa and dura for all experimental groups together (circle, saline; square, formalin; triangle, capsaicin).1
Fig.2 The inflammation was quantified by spectrophotometric measurement of formamide extracts of Evans blue dye (ng of the dye per mg of tissue in the nasal mucosa (A) and dura mater (B). (C) Individual animal values are plotted showing extravasation in the nasal mucosa and dura for all experimental groups together (circle, saline; square, formalin; triangle, capsaicin).1
Reference
- Lovrenčić, Luka et al. "Association of Intranasal and Neurogenic Dural Inflammation in Experimental Acute Rhinosinusitis." Frontiers in pharmacology vol. 11 586037. https://doi.org/10.3389/fphar.2020.586037. Distributed under Open Access license CC BY 4.0, with modification.
For Research Use Only.
