Delayed Type Hypersensitivity (DTH) Modeling & Pharmacodynamics Services
Creative Biolabs offers a comprehensive range of well-established DTH models, offering precise and reliable platforms to evaluate the pharmacological effects of immune-targeting drugs, vaccines, and novel therapeutic candidates efficiently. These models are designed to ensure reproducible and high-quality results for preclinical studies.
Introduction
Delayed Type Hypersensitivity (DTH) is a form of T-cell-mediated immune response that typically develops 24–72 hours after exposure to a specific antigen. Unlike immediate hypersensitivity reactions that rely on antibodies, DTH is primarily driven by the activation of CD4+ T-helper cells, which recruit and activate macrophages at the site of antigen exposure, resulting in localized inflammation. This immune mechanism is central to the pathogenesis of various autoimmune and inflammatory diseases, such as contact dermatitis, rheumatoid arthritis, multiple sclerosis, and certain infectious diseases like tuberculosis. DTH responses are also crucial for understanding transplant rejection and evaluating vaccine induced cellular immunity. The model reproduces key features of human immune reactions, including cytokine release, immune cell infiltration, and tissue swelling, making it a valuable tool for immunological research. Researchers rely on DTH models to study the mechanisms of cell-mediated immunity, investigate disease pathology, and screen the efficacy of immunomodulatory or anti-inflammatory therapeutics.
Delayed Type Hypersensitivity Model and Applications
Creative Biolabs offers a wide range of well-established rodent models for Delayed Type Hypersensitivity (DTH), simulating immune-mediated diseases such as autoimmune conditions and transplant rejection. These models are designed to accurately replicate cell-mediated immune responses and are coupled with comprehensive evaluations, enabling precise assessment of drug candidates aimed at modulating immune responses. Our team of expert scientists will guide you through the entire process, from experimental design to data analysis, ensuring that you receive high-quality, reliable results. For more information about our DTH models for preclinical research, please explore the links below:
| DTH Models | Simulates | Evaluates Drugs | Animal species |
| Chemical induced Rodent Contact Hypersensitivity Models | T-cell mediated immune response leading to localized inflammation, erythema, and edema at the site of antigen challenge. | Anti-inflammatory drugs (e.g., corticosteroids), immune modulators (e.g., anti-TNFα), and T-cell inhibitors (e.g., cyclosporine). | Mouse |
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a range of measurements for evaluating drug efficacy in Delayed Type Hypersensitivity models, utilizing a variety of advanced technologies, including:- General observations: inflammation severity, erythema, and edema at the site of antigen injection
- Histopathology: tissue sections for infiltration of T-cells, macrophages, and other immune cells
- Cytokine profiling (e.g., ELISA): levels of key inflammatory cytokines such as TNF-α, IFN-γ, and IL-12
- Flow cytometry: T-cell and macrophage activation and subpopulation analysis
- Gene/protein expression: analysis of immune response markers using RT qPCR and Western blotting
- Serum biomarkers: levels of acute phase proteins and inflammatory mediators
In addition to the established models, our team provides custom solutions based on specific research needs, ensuring accurate experimental design and robust data analysis throughout the study.
Our advantages
- Expertise: In-depth knowledge of immunology and disease modeling
- Customization: Tailored models to meet specific research objectives
- Advanced Technologies: Utilization of cutting-edge measurement tools for precise evaluation
- Comprehensive Support: End-to-end assistance, from experimental design to data interpretation
- Reliability: Proven models and methodologies ensuring reproducible results
- Flexibility: Ability to adjust models to simulate a variety of immune-related conditions
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. How long does it take to establish a Delayed Type Hypersensitivity Model?
The model typically takes 10–14 days to establish, including sensitization and antigen challenge. Additional monitoring may be required depending on the therapeutic intervention being evaluated.
-
2. Can this model be used for chronic inflammation studies?
While the DTH model is best suited for acute responses, it can be adapted for chronic inflammation studies by multiple antigen challenges and monitoring prolonged immune responses.
-
3. Is there a specific animal model that is better suited for DTH?
Mice are most commonly used for this model, but rats and other species can also be utilized depending on the research needs.
-
4. How do you measure the effectiveness of a drug in this model?
Drug effectiveness is assessed through a combination of clinical signs of inflammation, histological analysis, cytokine profiling, and immune cell activation markers.
-
5. Can this model be used to test both biological and small-molecule drugs?
Yes, the Delayed Type Hypersensitivity Model can evaluate both biological agents, like monoclonal antibodies, and small molecules targeting immune responses.
Published Data
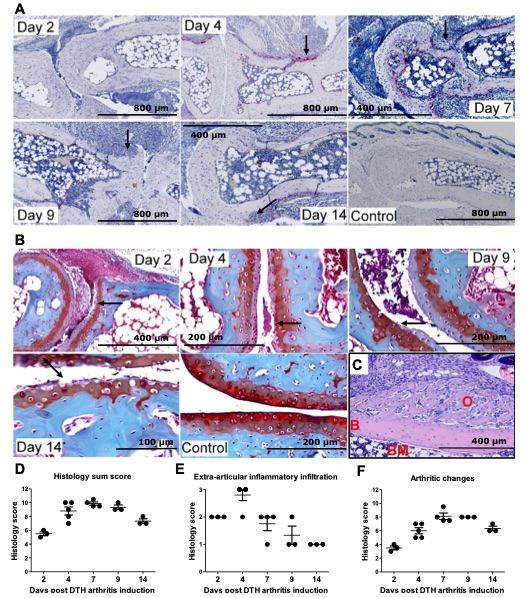 Fig.1 Delayed-type hypersensitivity model with arthritic manifestations in C57BL/6J mice.1
Fig.1 Delayed-type hypersensitivity model with arthritic manifestations in C57BL/6J mice.1
In this study, we aim to establish the DTH arthritis model in female C57BL/6 mice. Histopathological analyses were performed to evaluate the arthritic pathology in the DTH-arthritis model, and a scoring system was applied (see Materials and methods for details). The evaluation revealed that, in the mBSA-challenged paw, the mice developed a severe arthritis and peri-articular inflammation characterized by influx of inflammatory cells, hyperplasia of the synovial membrane and pannus formation with the presence of fibroblast-like cells, increased (compared with unchallenged control animals) osteoclast activity (assessed by the increase in number of TRAP-positive cells, stained red, Figure 1a) and bone erosion, and cartilage destruction evidenced by loss of Safranin O staining (Figure 1b). The arthritis was localized in the ankle, tar- sus, and metatarsophalangeal joints of the mBSA-challenged paw. Bone erosion was evident on day 4 after DTH-arthritis induction and afterwards, and cartilage destruction (assessed by loss of Safranin O staining of articular cartilage) was evident on day 2 after DTH- arthritis induction and afterwards. Osteoclast number was reduced by day 14 after DTH-arthritis induction compared with days 4, 7, and 9, but no repair of the damage to bone integrity was apparent. However, new bone formation was observed and was most prevalent as osteophytes adjacent to the affected joints of the paw (Figure 1c). The histology sum score reached a peak on day 7 after arthritis induction (Figure 1d), and the extra- articular inflammation peaked on day 4 after arthritis induction (Figure 1e). The sum score for arthritic changes (calculated as the total sum score minus the score for extra-articular inflammation) reached its peak on days 7 to 9 after arthritis induction (Figure 1f).
Reference
- Atkinson, Sara M et al. "Establishment and characterization of a sustained delayed-type hypersensitivity model with arthritic manifestations in C57BL/6J mice." Arthritis Research & Therapy vol. 14,3 R134. 7 Jun. 2012. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1186/ar3867
For Research Use Only.
