Experimental Autoimmune Myasthenia Gravis (EAMG) Modeling & Pharmacodynamics Services
Creative Biolabs offers various fully developed models for the assessment of drug efficacy in treating EAMG, allowing for the evaluation of immunotherapies, neuromuscular agents, and other treatments targeting autoimmune neuromuscular diseases.
Introduction
Experimental Autoimmune Myasthenia Gravis (EAMG) is a widely used preclinical model that simulates the autoimmune neuromuscular disorder, Myasthenia Gravis (MG). MG is a chronic condition in which the immune system produces antibodies that attack acetylcholine receptors (AChR) at the neuromuscular junction, impairing communication between the nerve and muscle. This disruption leads to muscle weakness, fatigue, and in severe cases, respiratory failure. EAMG is induced in rodents by immunizing them with AChR or AChR peptides, leading to the production of autoantibodies that mimic the pathophysiology of MG. The model serves as a valuable tool for understanding the molecular mechanisms underlying the disease and evaluating therapeutic interventions.
EAMG Models and Applications
Creative Biolabs offers a range of well-established rodent models for studying Experimental Autoimmune Myasthenia Gravis (EAMG). These models are meticulously designed to replicate the pathophysiology of Myasthenia Gravis (MG), an autoimmune neuromuscular disorder characterized by the production of antibodies against acetylcholine receptors (AChR), leading to impaired neuromuscular transmission and muscle weakness. The models include both antigen induced and transgenic models that simulate the disease's progression, allowing for detailed evaluation of drug candidates and therapies targeting autoimmune neuromuscular diseases. Our experienced team will collaborate with you at every stage of your project, from experimental design to data analysis, ensuring reliable and accurate results. To learn more about the EAMG models available for preclinical research, please explore the links below:
| EAMG Models | Description | Evaluated Drugs | Animal species |
| Antigen induced EAMG Model | Induced by immunizing rodents with acetylcholine receptor (AChR) peptides or AChR protein. This model mimics the immune response in Myasthenia Gravis patients. | Immunosuppressants, acetylcholinesterase inhibitors, immunomodulators. | Rat, Mouse |
| Transgenic Mice Spontaneous EAMG Model | A transgenic model that spontaneously develops autoimmune myasthenia gravis symptoms, including muscle weakness and ptosis, without the need for external immunization. | Targeted therapies, gene therapies, and immune checkpoint inhibitors. | Mouse |
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in the EAMG model, utilizing advanced technologies such as:- General observations: Muscle strength testing, clinical scoring (ptosis, limb weakness), body weight changes.
- Electrophysiological tests: Compound muscle action potential (CMAP) amplitude to assess neuromuscular transmission.
- Serum analysis: Measurement of AChR antibody levels to monitor immune response.
- Histopathology: Evaluation of muscle fiber damage and inflammation in skeletal muscles.
- Cytokine profiling (e.g., ELISA): Levels of pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6.
- Gene/protein expression profiling: RT-qPCR and Western blot analysis to assess the expression of immune-related genes and proteins.
Our advantages
- Comprehensive Drug Evaluation: Allows for the testing of a wide range of therapeutic candidates, from immunosuppressants to neuromuscular agents.
- Advanced Analytical Tools: We use state-of-the-art methods for assessing disease progression and drug effects, ensuring high-quality data.
- Customized Services: Our team works closely with clients to tailor experimental designs and measurements to specific research needs.
- Experienced Scientific Support: Expert guidance throughout the project, from model selection to data analysis.
- Reliable and Reproducible Results: We ensure robust model performance to support reliable preclinical findings.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the typical duration for studying drug efficacy in the EAMG model?
The study duration varies depending on the drug being tested, but most efficacy studies last from 2 to 4 weeks.
-
2. What species are commonly used in this model?
The most common species are C57BL/6 mice and Lewis rats, which are suitable for inducing autoimmune responses.
-
3. Can this model be used to evaluate both preventive and therapeutic treatments?
Yes, the model is flexible and can be used to test both preventive and therapeutic drug candidates targeting autoimmune neuromuscular disorders.
-
4. What are the main limitations of the EAMG model?
While it closely mimics the autoimmune nature of Myasthenia Gravis, it does not fully replicate the long-term disease progression seen in human patients.
-
5. How do you ensure the reliability of the model?
Our models are standardized and validated through rigorous testing and reproducibility studies, ensuring consistent results for your research.
Published Data
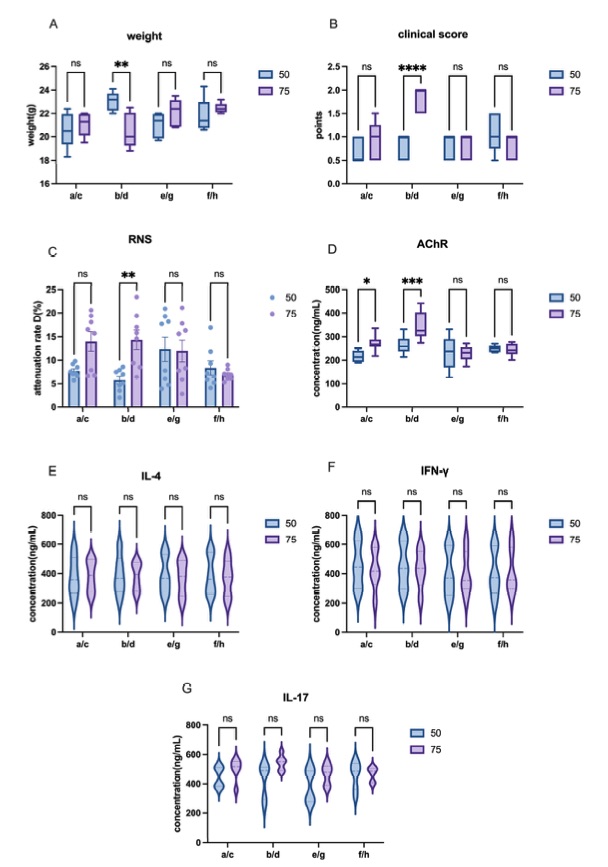 Fig. 1 Different doses of AChR influence on EAMG model induction of C57 mice.1
Fig. 1 Different doses of AChR influence on EAMG model induction of C57 mice.1
The study aimed to optimize and standardize the process of inducing the Experimental Autoimmune Myasthenia Gravis (EAMG) model by evaluating various induction conditions to improve the success rates. Female Lewis rats and C57BL/6 mice were used to compare their sensitivity to model induction. A controlled variable approach was employed, focusing on the dosages of acetylcholine receptor (AChR) and H37Ra, as well as factors such as mixing time and injection sites. In the case of C57 mice, model induction was unsuccessful; however, a comparison of different AChR dosages provided valuable insights. The group receiving 75 μg of AChR exhibited higher clinical scores and a more pronounced reduction in repetitive nerve stimulation (RNS) responses, which correlated with elevated AChR levels in several experimental groups (Figure 1A–D).
Reference
- Zhang, Xiangrui et al. "Optimization of Induction Protocols for Experimental Autoimmune Myasthenia Gravis." International Journal of Molecular Sciences vol. 26,10 4628. 12 May. 2025. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/ijms26104628
For Research Use Only.
