Systemic Lupus Erythematosus (SLE) Modeling & Pharmacodynamics Services
Creative Biolabs offers a variety of well-established animal models to evaluate the efficacy of drug candidates targeting SLE. These models provide valuable insights into disease pathophysiology, immune responses, and potential therapeutic interventions, contributing to the development of more effective treatments for this challenging disease.
Introduction
Systemic Lupus Erythematosus (SLE) is a complex, chronic autoimmune disease that affects multiple organs and systems, primarily targeting the skin, kidneys, joints, and heart. It is characterized by the body's immune system mistakenly attacking healthy tissue, leading to inflammation and organ damage. SLE can present with a wide range of symptoms, including fatigue, joint pain, skin rashes, and kidney dysfunction, making diagnosis and management challenging. The disease is more common in women, particularly during their reproductive years, and its exact cause remains unclear, though genetic, hormonal, and environmental factors are believed to play a role. SLE is marked by the production of autoantibodies, such as anti-dsDNA and anti-Smith, which contribute to immune complex formation and tissue injury. Despite significant advancements in treatment, there is still no cure, and current therapies focus on immunosuppression and symptom management.
SLE Models and Applications
Creative Biolabs offers a comprehensive selection of well-established rodent models for studying Systemic Lupus Erythematosus (SLE), including spontaneous SLE mouse models, induced models, and SLE patient PBMC reconstitution-lupus-like models. These models are carefully designed to replicate the autoimmune characteristics of human lupus, such as autoantibody production, renal damage, and systemic inflammation. They provide an ideal platform for evaluating potential therapeutic candidates during the preclinical phase. Our experienced team of scientists is dedicated to supporting your research from experimental design through to data analysis, ensuring accurate and reliable results. To learn more about the SLE models available for preclinical research, please explore the links below:
| Model | Simulates Disease | Evaluates Drugs | Animal species |
| Spontaneous Systemic Lupus Erythematosus (SLE) Mouse Models | Systemic Lupus Erythematosus (SLE) with autoimmune characteristics, including autoantibody production, renal damage, and systemic inflammation. | Immunosuppressive drugs (e.g., corticosteroids), biologics targeting B cells, TNF inhibitors, and novel immunotherapies. | Mouse |
| Induced Systemic Lupus Erythematosus (SLE) Models | Induced lupus with specific triggers such as pristane or LPS, showing similar symptoms to human SLE, including immune system dysregulation, skin rashes, and kidney involvement. | Anti-inflammatory drugs, immunosuppressants, biologic therapies, and potential new treatments for organ protection. | Mouse |
| SLE Patient PBMC Reconstitution-Lupus-Like Model | A lupus-like condition induced by reconstituting mice with peripheral blood mononuclear cells (PBMCs) from SLE patients, replicating human SLE symptoms. | Targeted therapies for immune modulation, therapies aimed at reducing autoantibody production, and novel biologics to regulate immune cell activity. | Mouse |
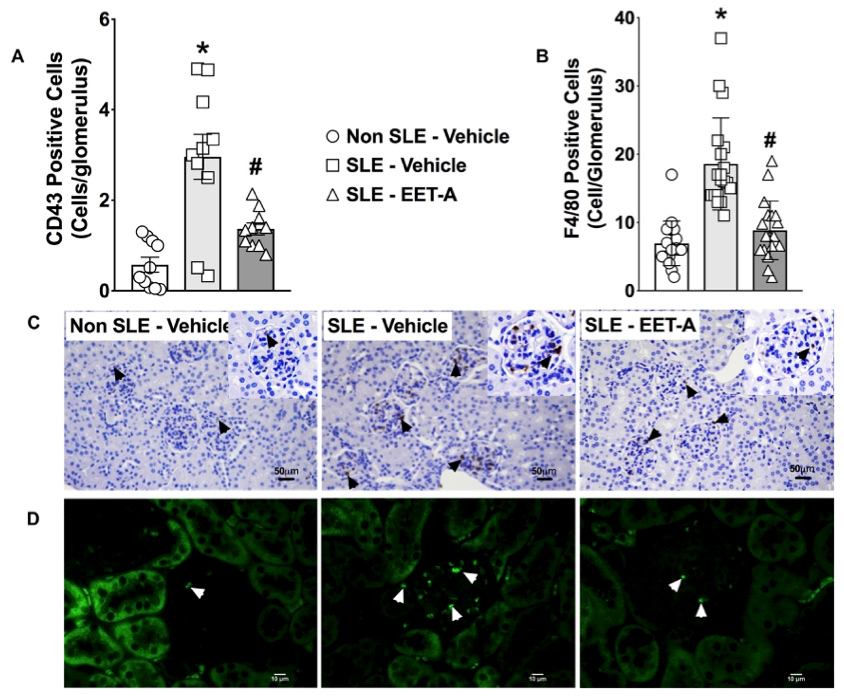 Fig. 1 Experimental results of the systemic lupus erythematosus (SLE) mouse model.1,3
Fig. 1 Experimental results of the systemic lupus erythematosus (SLE) mouse model.1,3
Evaluation Platform
- Animals: Mouse.
-
Measurements
We offer a range of measurements to evaluate drug efficacy in SLE models, utilizing advanced technologies and methods, including but not limited to:- General Observations: Disease onset, severity of symptoms, survival rate, and progression of kidney dysfunction.
- Immunohistochemistry: Infiltration of immune cells (e.g., T-cells, B-cells) in organs like kidneys, lungs, and skin.
- Cytokine Profiling (e.g., ELISA): Levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IFN-γ.
- Renal Function Tests: Serum creatinine, BUN, and urine protein to assess kidney damage.
- Antibody Titers: Measurement of autoantibodies, such as anti-dsDNA and anti-Smith antibodies, using ELISA.
- Flow Cytometry: Profiling of immune cell populations, including regulatory T cells and B cells, to assess immune balance.
- Histopathology: Tissue damage assessment using H&E and Masson's trichrome staining to evaluate organ involvement, especially in the kidneys.
Our expert team offers tailored experimental design, model selection, and data analysis, ensuring a precise and effective approach to your research needs.
Our advantages
- Expertise and Customization: Our team offers expert guidance in model selection, experimental design, and data analysis.
- Reliable Disease Modeling: Well-established murine strains and induced models faithfully replicate human SLE pathology.
- Advanced Technologies: Utilization of cutting-edge methods such as immunohistochemistry, cytokine profiling, and flow cytometry to provide detailed insights.
- Versatility: Tailored approaches for both acute and chronic disease models.
- Comprehensive Services: From drug screening to biomarker evaluation, we cover all aspects of SLE research.
- Preclinical Research Support: Supporting the transition from bench to clinic by evaluating potential lupus therapeutics in a controlled, reproducible environment.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the typical age at which SLE develops in the model?
The disease typically begins to manifest between 12-16 weeks in most murine models, mirroring the age of onset in human patients.
-
2. Can this model be used for long-term studies?
Yes, both acute and chronic forms of SLE can be studied, with models designed for long-term observation of disease progression and treatment outcomes.
-
3. Are the models suitable for testing combination therapies?
Absolutely. Our SLE models are ideal for evaluating the synergistic effects of combination therapies, such as biologics combined with traditional immunosuppressants.
-
4. How do you ensure consistency in model development?
We use well-characterized, genetically predisposed strains and follow standardized protocols to ensure reproducibility and consistency across experiments.
-
5. What kind of biomarkers can be measured in this model?
Biomarkers such as anti-dsDNA, anti-Smith antibodies, cytokine levels, and renal function markers are routinely measured to assess disease activity and therapeutic response.
Published Data
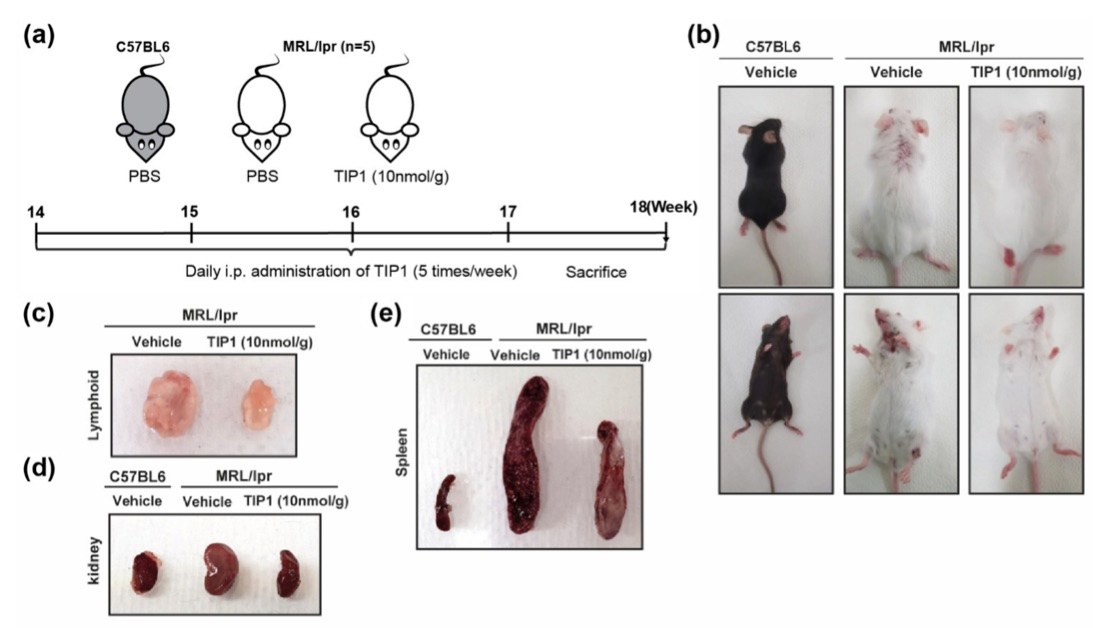 Fig. 2 The dramatic inhibitory effect of Toll-like receptor inhibitor peptide 1 (TIP1) on systemic lupus erythematosus (SLE) in a mouse model. 2,3
Fig. 2 The dramatic inhibitory effect of Toll-like receptor inhibitor peptide 1 (TIP1) on systemic lupus erythematosus (SLE) in a mouse model. 2,3
To evaluate the therapeutic potential of TIP1, it was administered to B6J and MRL/lpr mice. Since MRL/lpr mice spontaneously develop systemic lupus erythematosus (SLE) over a few months, treatment was initiated when the mice reached 14 weeks of age. TIP1 was injected intraperitoneally five times per week for 4 weeks (Figure 2a). Dorsal and abdominal skin images were captured to observe any changes in the overall appearance of the mice. TIP1 treatment significantly reduced skin damage in the MRL/lpr mice, resulting in a healthier appearance compared to those treated with the vehicle (Figure 2b). The sizes of major organs, including the kidney, spleen, and lymph nodes, were also examined. While these organs were significantly enlarged in the vehicle-treated MRL/lpr mice, TIP1 administration notably reduced the sizes of the kidney, spleen, and lymph nodes (Figure 2c–e). These results suggest that TIP1 effectively mitigated the progression of nephromegaly, splenomegaly, and lymphadenopathy in MRL/lpr mice.
References
- Hye Khan, Md Abdul et al. "Epoxyeicosatrienoic Acid Analog EET-A Blunts Development of Lupus Nephritis in Mice." Frontiers in Pharmacology vol. 10 512. 10 May. 2019. https://doi.org/10.3389/fphar.2019.00512
- Baek, Wook-Young et al. "Toll-like Receptor Signaling Inhibitory Peptide Improves Inflammation in Animal Model and Human Systemic Lupus Erythematosus." International Journal of Molecular Sciences vol. 22,23 12764. 25 Nov. 2021. https://doi.org/10.3390/ijms222312764
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
