Pulmonary Edema Modeling & Pharmacodynamics Services
Introduction
Pulmonary edema is a condition characterized by abnormal fluid accumulation in the alveolar spaces, which disrupts normal oxygenation and ventilation. It is classified into cardiogenic and non-cardiogenic pulmonary edema, significantly contributing to morbidity and overall mortality in critically ill patients. The acute respiratory insufficiency it causes is considered a life-threatening emergency. Patients with pulmonary edema experience prolonged hospital stays and extended durations of mechanical ventilation, complicating their hospital course. Its impact is particularly pronounced in patients with primary respiratory failure, those undergoing transplant surgery or cardiothoracic surgery, and individuals with traumatic brain injury. Creative Biolabs has established a set of pulmonary edema models according to the clinicopathological features of cardiogenic and non-cardiogenic pulmonary edema. It is used to facilitate preclinical research and development of pharmacology and efficacy, as well as studies on pathological mechanisms.
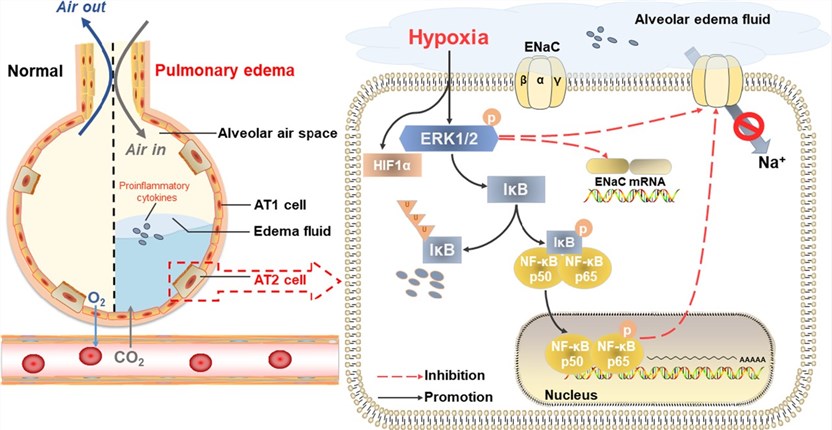 Fig.1 The schematic diagram depicts the potential mechanism for the decreased expression of ENaC in AT2 cells during hypoxia.1
Fig.1 The schematic diagram depicts the potential mechanism for the decreased expression of ENaC in AT2 cells during hypoxia.1
Available Pulmonary Edema Models
| Pulmonary Edema Models | Clinical Relevance | Primary Research Applications | Animal Species |
| LPS-Induced Pulmonary Edema Model | Acute Respiratory Distress Syndrome (ARDS), sepsis, and acute lung injury (ALI). | Endothelial Protective Agents (to restore vascular barrier function), Anti-inflammatory Drugs (e.g., TLR4 antagonists), and broad-spectrum ARDS Therapeutics. | Mouse, Rat |
| Unilateral Nephrectomy & DOCA-Induced Nephrogenic Pulmonary Edema Model | Cardiorenal syndromes, hypertension, and renin-angiotensin-aldosterone system (RAAS) dysregulation. | Cardiorenal Therapeutics (targeting volume overload and hypertension), Diuretics (e.g., Furosemide), and RAAS Blockers (e.g., ACE inhibitors like Enalapril or ARBs). | Rat |
| Epinephrine/Norepinephrine-Induced Acute Cardiogenic Pulmonary Edema Model | Neuro-humoral dysfunction and acute cardiac failure (triggered by massive sympathetic overactivation). | β-Blockers (to stabilize cardiac function during acute stress, e.g., Metoprolol), and other Neuro-humoral Axis Modulators. | Mouse, Rat |
Evaluation Platform
Our state-of-the-art platforms are the foundation of our robust quality control, delivering validated models and ensuring the superior accuracy of all pharmacodynamic evaluations to accelerate your drug pipeline. To achieve this, we utilize the following specialized platforms:
- Pulmonary function analysis: This platform assesses pulmonary gas exchange and ventilation function, as well as the degree of lung tissue damage.
- Inflammation detection: This platform evaluates the levels of inflammatory factors, chemokines, cell populations, and the proportion of inflammatory cells in bronchoalveolar lavage fluid (BALF) or serum.
- Pathway analysis: This platform uses molecular biology and omics technologies to identify and evaluate pathways related to pulmonary edema, such as those involved in oxidative stress and permeability.
- Imaging analysis: This platform performs lung tissue pathology and in vivo imaging.
- In Vitro experiment: This platform simulates pulmonary edema-related pathological features by inducing cell damage through hypoxia, lipopolysaccharide (LPS), hypertonicity, or inflammatory factors. It also utilizes ex vivo perfused mouse, rat, or dog lung models, as well as cell co-culture models.
Applications
- Clinical Disease Modeling: These models precisely replicate clinical hydrostatic (cardiogenic/renal failure) and permeability (ARDS/Sepsis) edema, ensuring the preclinical platform is perfectly aligned with major human etiologies. This provides a focused, high-relevance system for developing type-specific anti-edema therapies.
- Mechanistic Studies: The models are essential for dissecting the molecular basis of fluid accumulation. Research focuses on validating novel targets by precisely investigating endothelial barrier integrity, the function of alveolar fluid clearance systems (ENaC, Aquaporins), and the role of neuro-humoral dysregulation (e.g., RAAS) in edema initiation and resolution.
- Preclinical PD Assessment: These models are indispensable for rigorous PD studies, offering direct, quantitative assessment of a drug's in vivo efficacy. By measuring the compound's ability to reverse fluid accumulation and modulate inflammation under pathological stress, these models are essential for establishing crucial dose-response relationships and accelerating therapies toward clinical translation.
Our advantages
- Extensive Model Library: We have developed standardized pulmonary edema models for various etiologies, including cardiogenic, inflammatory, high-altitude/hypoxia-induced, nephrogenic, and drug-induced edema. These models are rigorously validated to reflect clinical characteristics.
- Multi-Dimensional Evaluation System: Our approach combines functional, morphological, molecular biology, omics, and imaging systems to verify if models accurately reflect the core characteristics of clinical pulmonary edema. This also helps us identify new targets and supports pharmacological efficacy research.
- Rich Experience: Our team comprises experts in respiratory physiology, pathology, molecular biology, and other relevant fields. We offer scientific consulting services to help clients optimize model design and precisely interpret results. Our extensive pulmonary edema model database also helps clients quickly identify experimental abnormalities, boosting research efficiency.
- Standardized SOP Procedures: We have established Standard Operating Procedures (SOPs) for the entire process, from model design and creation to sample testing and data delivery. This significantly shortens project cycles.
- Customized Solution Design: We tailor model creation methods, detection indicators, and data analysis based on client needs. For instance, for anti-inflammatory drug development, we can design a solution involving LPS induction with dynamic inflammatory factor monitoring.
- One-Stop Service: Beyond pulmonary edema model services, we also offer models for other respiratory system diseases, autoimmune diseases, and digestive system diseases, as well as toxicology evaluations.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What are the core advantages of your models compared to those of other institutions?
A: Our pulmonary edema models boast strong clinical relevance, as their pathological features, such as lung water content and inflammatory cytokine profiles, highly match human patient data based on clinical comparisons. Furthermore, we ensure controllable stability by minimizing human error and guaranteeing high model success rates through adherence to standardized SOPs and stringent internal quality control (QC).
-
Q: For rare types of pulmonary edema, such as high-altitude pulmonary edema, what are the unique features of your modeling technology?
A: We use step-wise hypobaric regulation technology to simulate the actual rate of altitude ascent, equipped with a real-time blood gas monitoring module. This allows us to dynamically track the process and simultaneously monitor animal cardiac function, thereby replicating the multi-organ pathological features of high-altitude pulmonary edema.
-
Q: If our drug targets a specific signaling pathway, can your company customize a model to validate its relevance to the target?
A: Yes, absolutely. Leveraging our gene-engineered animal model platform, we can provide: gene knockout/knock-in models, conditional activation models, and humanized animal models for specific targets. Alternatively, based on the information you provide, we can select animal species with strong target cross-reactivity.
-
Q: How to adjust the model protocol according to the stages of drug development, such as early screening and candidate drug evaluation?
A: We provide phased customization services tailored to your drug development stage, starting with early screening using high-throughput mouse models that focus on core indicators like lung water content for rapid elimination of ineffective compounds (supporting over 50 samples in a single run). For candidate drug evaluation, we upgrade the model to include additional functional indicators, quantitative histopathological analysis, and pharmacokinetic-pharmacodynamic correlation analysis. Finally, for preclinical IND filing, we strictly adhere to all relevant regulations and animal welfare ethics, providing complete model validation data, original raw data, and animal ethics review documents to support your submission.
-
Q: How do you ensure the objectivity and reproducibility of data?
A: We employ a full-process quality control system. This includes using SPF-grade standardized strains and ensuring all data undergoes double-blind review. We also support the traceability of raw data to comply with electronic data standards for project submissions.
Published Data
Dexmedetomidine (Dex) relieves pulmonary edema in the LPS-induced ALI model by activating the PI3K pathway, leading to both inflammation suppression and enhanced alveolar fluid clearance (AFC) function. This illustrates the capability of the LPS-induced pulmonary edema model for preclinical R&D.
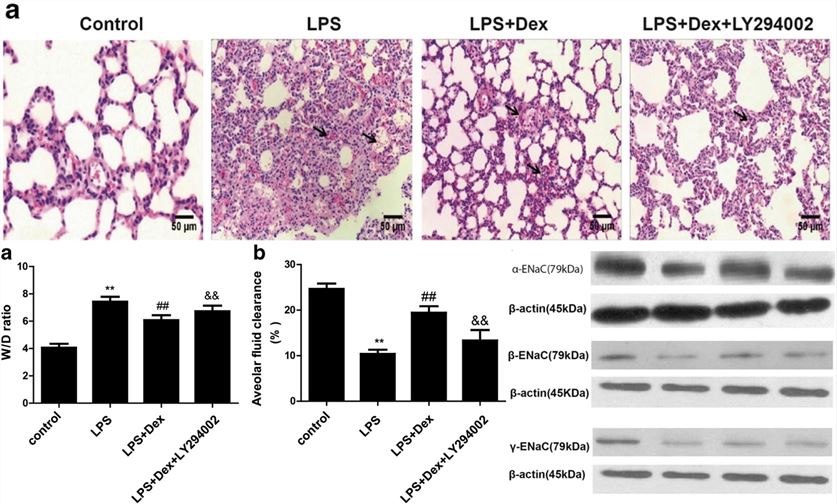 Fig.2 Effects of Dex on lung pathology, ENaC protein expression, lung wet-to-dry weight (W/D) ratio, and alveolar fluid clearance (AFC) in LPS-induced pulmonary edema model.2
Fig.2 Effects of Dex on lung pathology, ENaC protein expression, lung wet-to-dry weight (W/D) ratio, and alveolar fluid clearance (AFC) in LPS-induced pulmonary edema model.2
References
- Zhou, Wei et al. "Submersion and hypoxia inhibit alveolar epithelial Na+ transport through ERK/NF-κB signaling pathway." Respiratory research vol. 24,1 117. https://doi.org/10.1186/s12931-023-02428-z. Distributed under Open Access license CC BY 4.0, without modification.
- Jiang, Yuanxu et al. "Dexmedetomidine alleviates pulmonary edema through the epithelial sodium channel (ENaC) via the PI3K/Akt/Nedd4-2 pathway in LPS-induced acute lung injury." Immunologic research vol. 69,2 (2021): 162-175. https://doi.org/10.1007/s12026-021-09176-6. Distributed under Open Access license CC BY 4.0, with modification.
For Research Use Only.
