Passive Cutaneous Anaphylaxis (PCA) Modeling & Pharmacodynamics Services
At Creative Biolabs, we offer a range of well-established PCA models that can be tailored to your specific research needs, enabling comprehensive drug efficacy evaluations and contributing to advancements in allergy treatment.
Introduction
Passive Cutaneous Anaphylaxis (PCA) is a widely used animal model to study IgE-mediated allergic reactions, a key mechanism in Type I hypersensitivity. This model involves the passive sensitization of rodents with allergen-specific IgE antibodies, followed by exposure to the corresponding allergen, which triggers local allergic responses such as erythema, edema, and increased vascular permeability. PCA is valuable for evaluating drug efficacy in treating allergic diseases like asthma, rhinitis, and anaphylaxis. The model helps to examine immune responses, including mast cell activation, cytokine release, and tissue inflammation, which are central to allergic reactions. It provides a controlled environment to assess the effects of various therapeutic agents, including antihistamines, corticosteroids, and biologics targeting IgE or its receptors. By understanding these immune processes, PCA contributes to the development of novel allergy therapies and allows researchers to evaluate the safety and efficacy of potential drugs in preclinical studies.
Passive Cutaneous Anaphylaxis Model and Applications
Creative Biolabs offers a comprehensive range of well-established rodent models for passive cutaneous anaphylaxis (PCA) research. This model is widely used to simulate IgE-mediated allergic reactions, including those found in conditions like allergic rhinitis, asthma, and anaphylaxis. The PCA model is induced by sensitizing animals with an allergen (such as ovalbumin or dinitrophenyl) and then challenging them with an antigen, leading to localized inflammatory responses and increased vascular permeability. This model is highly valuable for studying immediate-type hypersensitivity reactions and for evaluating the efficacy of therapeutic candidates aimed at managing allergic responses. To learn more about the PCA models available for preclinical research, please explore the links below:
| PCA Model | Simulates | Evaluates Drugs | Animal species |
| Anti-DNP-IgE Antibody induced Urticaria Model | IgE-mediated allergic responses, including localized inflammation, redness, and edema due to antigen-antibody interaction. | Antihistamines, mast cell stabilizers, leukotriene inhibitors, corticosteroids, and monoclonal antibodies (e.g., anti-IgE). | Mouse |
Evaluation Platform
- Animals: Mouse
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in this model, using advanced technologies, including:- General Observations: Reaction severity, skin erythema, and edema.
- Histology: Analysis of mast cell degranulation, immune cell infiltration, and tissue damage.
- Cytokine Profiling (e.g., ELISA): Quantification of inflammatory cytokines such as IL-4, IL-13, TNF-α, and histamine.
- Gene/Protein Expression: RT-qPCR and Western blot to assess gene and protein levels of immune markers (e.g., IgE, FcεRI).
- Vascular Permeability: Measurement of Evans blue dye leakage to assess vascular integrity and permeability.
In addition to established protocols, our scientific team can tailor the model to specific research needs, ensuring optimal experimental design and data analysis throughout your project.
Our advantages
- Customizable Models: We can adapt the model to meet specific research objectives, allowing for precise drug testing and evaluation.
- Advanced Data Analysis: Our team provides expert assistance with data interpretation and experimental design, ensuring high-quality results.
- State-of-the-Art Technologies: We utilize cutting-edge technologies, including cytokine profiling, histology, and gene expression analysis, to provide comprehensive data.
- Reliable Results: Our models are meticulously designed to offer reproducible and accurate outcomes, making them ideal for preclinical testing.
- Comprehensive Support: From model development to data analysis, our scientific team offers full support at every stage of your research.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What diseases can this model simulate?
This model primarily simulates IgE-mediated allergic reactions such as asthma, rhinitis, and food allergies.
-
2. What kind of drugs can be tested using this model?
Drugs such as antihistamines, corticosteroids, mast cell stabilizers, and monoclonal antibodies targeting allergic pathways can be tested for efficacy.
-
3. How is the PCA model established in rodents?
Rodents are sensitized with allergen-specific IgE antibodies, followed by antigen exposure to induce a localized allergic response, typically in the skin.
-
4. What measurements do you offer to assess drug efficacy?
We offer a variety of measurements, including general observations, histology, cytokine profiling, gene/protein expression analysis, and vascular permeability assessment.
-
5. Can the model be customized for specific research needs?
Yes, our models can be tailored to fit the specific requirements of your research, including adjustments in sensitization protocols and measurement techniques.
-
6. What are the advantages of using this model for preclinical testing?
This model provides a reliable and reproducible way to assess drugs that target IgE-mediated allergic responses, offering valuable insights for the development of allergy therapies.
Published Data
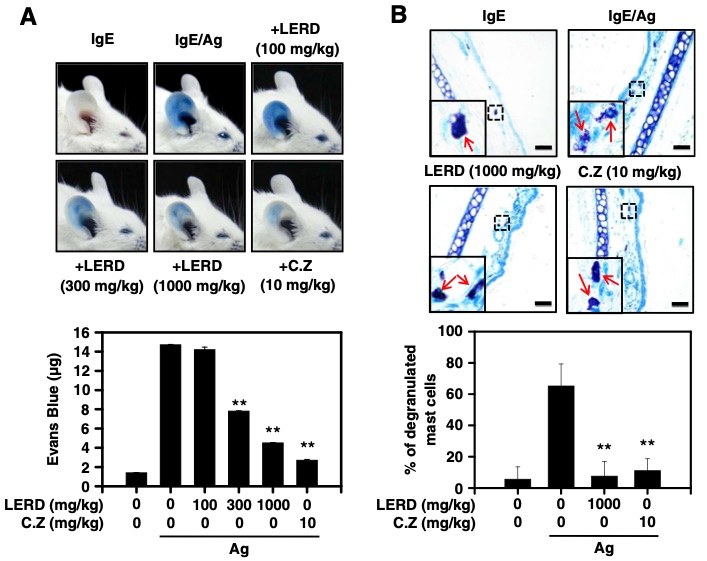 Fig. 1 LERD suppresses passive cutaneous anaphylaxis in vivo.1
Fig. 1 LERD suppresses passive cutaneous anaphylaxis in vivo.1
The anti-allergic effects of the leaf extract of Rhamnus davurica (LERD) were evaluated in mast cell cultures and a passive cutaneous anaphylaxis (PCA) animal model. The PCA model was induced in BALB/c mice to assess the in vivo anti-allergic activity of LERD. To investigate its effects, histological analysis was conducted on ear tissues to determine whether LERD inhibited mast cell degranulation. The PCA response was successfully triggered by the injection of IgE/antigen into the mice. Treatment with LERD resulted in a significant dose-dependent reduction of the allergic response, as shown in Figure 1A. Furthermore, histological analysis revealed that LERD effectively suppressed mast cell degranulation in the ear tissues following antigen exposure, as illustrated in Figure 1B. These findings suggest that LERD exerts an anti-allergic effect in vivo, primarily by inhibiting mast cell activation and degranulation.
Reference
- Kim, Ji Hyung et al. "Rhamnus davurica leaf extract inhibits Fyn activation by antigen in mast cells for anti-allergic activity." BMC complementary and alternative medicine vol. 15 80. 25 Mar. 2015. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1186/s12906-015-0607-6
For Research Use Only.
