COPD Modeling & Pharmacodynamics Services
Introduction
Chronic obstructive pulmonary disease (COPD), the third leading cause of death globally, is characterized by high morbidity and mortality rates, which are expected to increase due to aging populations and continuous exposure to risk factors like detrimental gases or particles, air pollution, and occupational hazards. Extended cigarette smoking, which induces irreversible airway remodeling and markedly diminishes lung function, constitutes a major risk factor for COPD. To bridge basic research and clinical translation, Creative Biolabs has established two types of COPD models to recapitulate key pathological features. These well-validated models enable controlled investigation of disease mechanisms and progression, accelerate target discovery, drug efficacy/safety evaluation, and biomarker validation, critical for advancing precision medicine in COPD.
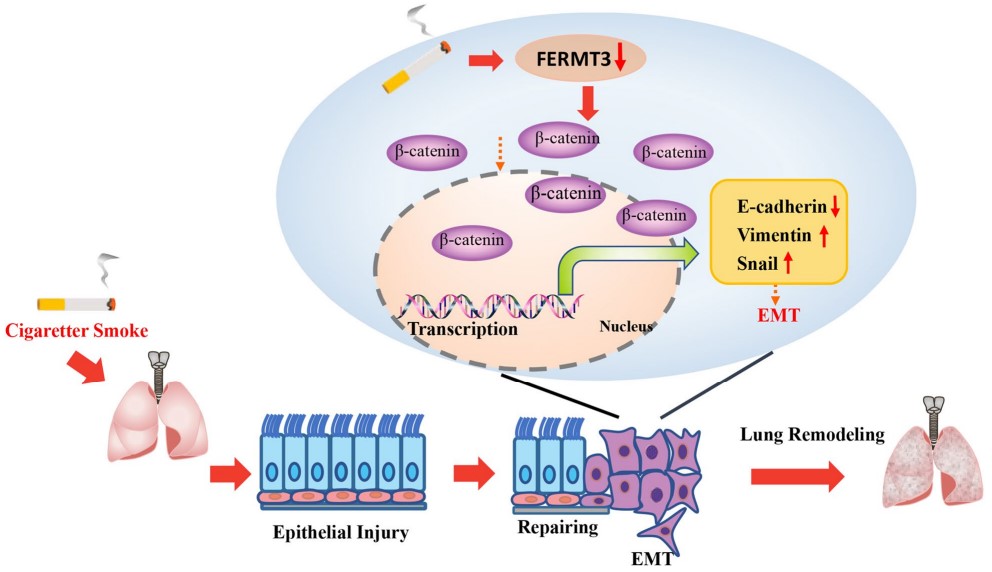 Fig.1 The mechanism of cigarette smoke (CS) promotes epithelial-mesenchymal transition in alveolar epithelial cells.1,5
Fig.1 The mechanism of cigarette smoke (CS) promotes epithelial-mesenchymal transition in alveolar epithelial cells.1,5
Available COPD Models
COPD models are tailored to different research objectives, such as rodent models (mice, rats, guinea pigs) for quick screening, non-human primates for translational relevance, and porcine models for large-animal physiological mimicry.
| Model | Rationale | Research Objective | Species |
| Cigarette Smoke-Induced COPD Model | Chronic COPD (with emphysema and airway remodeling), PM, and long-term smoke exposure-related pulmonary fibrosis and sustained oxidative damage. | Anti-fibrotic agents (e.g., Nintedanib), Antioxidants (e.g., N-acetylcysteine NAC), and Anti-inflammatory drugs (e.g., the PDE4 inhibitor Roflumilast). | Mouse, Rat |
| Cigarette Smoking & LPS-Induced Model | Acute Exacerbation of COPD (AE-COPD), specifically infection-triggered inflammatory storms and mucus plug formation. | Novel Anti-inflammatory drugs, Antibiotics (e.g., Azithromycin, which has immunomodulatory properties), Mucolytic agents (e.g., Ambroxol or other expectorants), and drugs targeting airway hypersecretion. | Mouse, Rat |
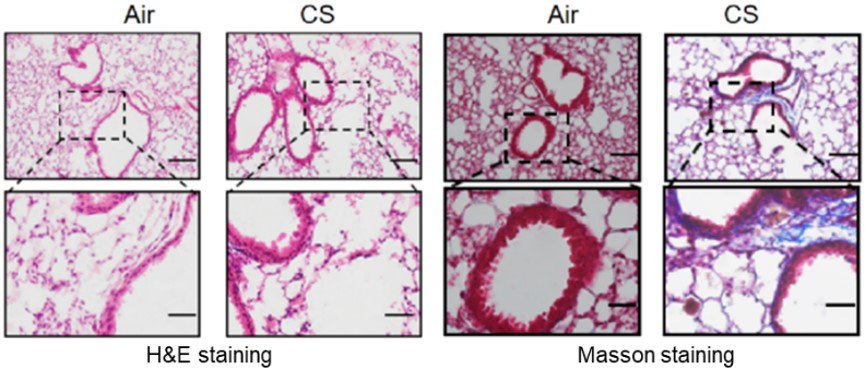 Fig.2 Lung Histopathology of CS-Induced Model.2
Fig.2 Lung Histopathology of CS-Induced Model.2
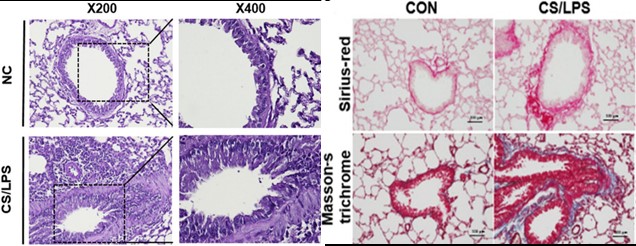 Fig.3 Lung Histopathology of CS & LPS-Induced Model.3
Fig.3 Lung Histopathology of CS & LPS-Induced Model.3
Evaluation Platform
We have established comprehensive detection platforms to gain a deeper understanding of COPD pathogenesis and drug mechanisms.
- Pathological Detection: We perform pathological examinations, including routine histology, immunohistochemistry (IHC), and immunofluorescence (IF), to observe pulmonary tissue changes, the expression, and localization of specific proteins.
- Pulmonary Function Testing: We evaluate animal lung function indicators and assess the degree of airway obstruction.
- Inflammation and Immunity Detection: We use inflammatory cytokine levels and immune cell subsets to assess changes in the immune response.
- Metabolomics and Proteomics: Our platform includes comprehensive metabolomics and proteomics analysis.
- Small Animal Imaging: We use Micro-CT, positron emission tomography (PET), and single-photon emission computed tomography (SPECT) to observe structural changes, as well as the metabolic and functional status of the lungs.
- Cell and Molecular Biology: We utilize gene editing to explore gene function, and employ RT-PCR to quantify gene expression levels.
Applications
- Clinical Relevance: Directly models chronic smoking exposure, leading to the development of chronic bronchitis, small airway remodeling, and emphysema.
- Mechanistic Studies: Primarily used to investigate the protease-antiprotease imbalance (e.g., MMPs and elastase) leading to alveolar destruction. Studies focus on structural changes and chronic inflammation.
- Drug Screening: Essential for evaluating therapies aimed at protecting alveolar structure, inhibiting elastase activity, or alleviating chronic inflammation (e.g., anti-inflammatory drugs, antiproteases).
Our advantages
- Comprehensive COPD Research Solutions: We offer a diverse range of COPD models, including acute/chronic CS-induced models (with or without LPS), and genetic models, which precisely mimic various pathological phenotypes of COPD.
- High Success Rate: Our standardized protocols and rigorous QC ensure a high success rate (exceeding 90-95%) and excellent reproducibility, thereby minimizing experimental variability.
- End-to-end service: Our end-to-end service covers everything from model design, induction, and sample analysis to data interpretation.
- Customized model: We offer customization capabilities, including CS exposure duration for disease stages or comorbidity co-induction to address your specific research questions.
- Professional team: Our dedication to scientific rigor is demonstrated through strict adherence to Good Laboratory Practice (GLP) standards, the application of power analysis, and the inclusion of sham and control groups, all of which ensure data validity for regulatory compliance, supported by an interdisciplinary team comprising pulmonologists, pharmacologists, immunologists, and bioinformaticians focused on optimizing study design and data interpretation.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: How flexible are your COPD models for specific research needs?
A: Models are highly adaptable. We adjust induction protocols, such as CS exposure duration, elastase dosage, and incorporate comorbidities, like pulmonary hypertension, upon request.
-
Q: Which platforms are prioritized for drug efficacy evaluation?
A: Our key platforms for drug efficacy evaluation include lung function assessment, histopathology, immunological assays, and RNA-seq for pathway analysis.
-
Q: How do you validate translational relevance to human COPD?
A: We compare endpoints, such as cytokine profiles and lung function, with human data from GEO databases and clinical studies.
-
Q: Can models recapitulate AECOPD?
A: Yes. AECOPD is induced via secondary challenges (rhinovirus, H. influenzae) to mimic viral/bacterial triggers, with markers like IL-1β and sputum neutrophilia.
-
Q: Do you provide data analysis beyond raw data?
A: Yes, we conduct statistical analysis or descriptive analysis on the generated data, and comprehensive multi-omics integration, all delivered in an in-depth report.
Published Data
This example demonstrates the potential of the CS-induced model to accurately replicate human COPD pathological features and its application in drug R&D: In the CS group, lung tissues exhibited significant inflammatory cell infiltration (blue arrows) and alveolar structure destruction, which were significantly reduced by moderate and high doses of Progesterone (P4).
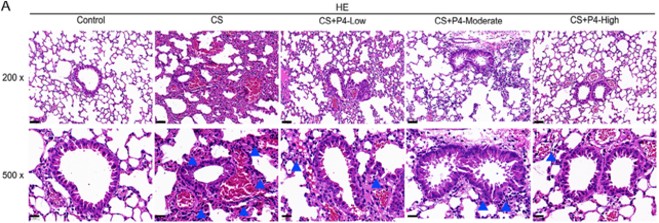 Fig.4 Histological changes observed in lung tissues.4,5
Fig.4 Histological changes observed in lung tissues.4,5
References
- Su, Xiaoshan et al. "FERMT3 mediates cigarette smoke-induced epithelial-mesenchymal transition through Wnt/β-catenin signaling." Respiratory research vol. 22,1 286. https://doi.org/10.1186/s12931-021-01881-y.
- Ding, Yu et al. "GLUT3-mediated cigarette smoke-induced epithelial-mesenchymal transition in chronic obstructive pulmonary disease through the NF-kB/ZEB1 pathway." Respiratory research vol. 25,1 158. https://doi.org/10.1186/s12931-024-02785-3. Distributed under Open Access license CC BY 4.0, with modification.
- Baek, Eun Bok et al. "Anti-inflammatory effect of Gyeji-tang in a chronic obstructive pulmonary disease mouse model induced by cigarette smoke and lipopolysaccharide." Pharmaceutical biology vol. 60,1 (2022): 2040-2048. https://doi.org/10.1080/13880209.2022.2131841. Distributed under Open Access license CC BY 4.0, with modification.
- Xie, Bin et al. "Progesterone (P4) ameliorates cigarette smoke-induced chronic obstructive pulmonary disease (COPD)." Molecular medicine (Cambridge, Mass.) vol. 30,1 123. https://doi.org/10.1186/s10020-024-00883-y.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
