Blood Circulatory System Infection Modeling & Pharmacodynamics Services
Introduction
Infections of the blood circulatory system, such as sepsis and dengue fever, often precipitate a systemic inflammatory response syndrome (SIRS), which can progress to multiple organ dysfunction syndrome (MODS) and carries a mortality rate of 20% to 30% (sepsis) and 35% to 50% (septic shock). Creative Biolabs' animal models must accurately reflect clinical conditions, comprehensively covering pathogen colonization, host immune response, and subsequent organ damage. These models constitute an essential bridge between basic scientific discovery and clinical medicine, providing indispensable in vivo evidence for deciphering pathogen virulence mechanisms, accelerating the development of novel anti-infective agents, and formulating optimized therapeutic strategies.
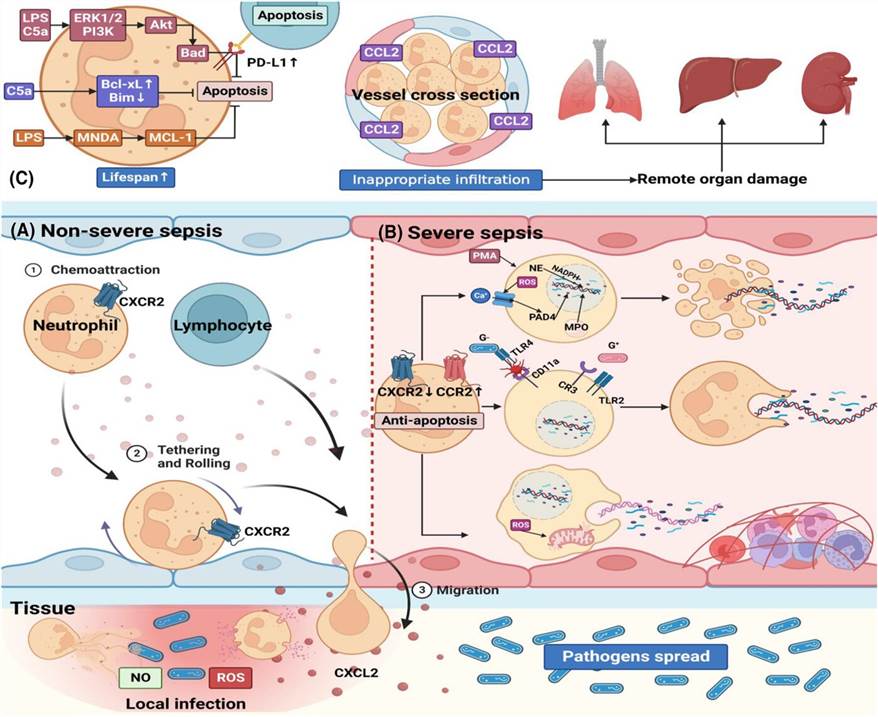 Fig.1 Molecular mechanisms and potential therapeutic targets of sepsis.1,3
Fig.1 Molecular mechanisms and potential therapeutic targets of sepsis.1,3
Available Blood Circulatory System Infection Models
Creative Biolabs has successfully established a diverse array of animal models for blood circulatory system infection diseases, utilizing methods such as intravenous injection, surgical implantation, and gene editing, to expedite diverse drug pharmacology and pharmacodynamic research and development, encompassing chemicals, biopharmaceuticals (e.g., antibodies and antibody-drug conjugates), cell therapies, nucleic acid-based therapeutics (NBTs), and vaccines.
| Blood CirculatorySystem Infection Models | Related Disease& Drug Evaluation | Animal Species |
| Dengue Viremia/Hemorrhagic Fever Models | Dengue Fever (DF), DHF, DSS; ideallysuited for the rigorous evaluation of Antivirals, Vaccine candidates, andImmune modulators. | Mouse,NHPs |
| EBOV Viremia/Systemic Models | Ebola Virus Disease (EVD), ViralHemorrhagic Fever (VHF); highly effective for the assessment of MonoclonalAntibodies and Antivirals (e.g., Remdesivir), Vaccine candidates. | Mouse,Guinea Pig, NHPs |
| Yellow Fever Viremia/Systemic Models | Yellow Fever, Viral Hemorrhagic Fever(VHF), specifically engineered for the evaluation of Antivirals (e.g., AT-752) andVaccine efficacy. | Hamster,Mouse, NHPs |
| CHIKV Viremia/Systemic Models | Chikungunya Fever (CHIKF), ChronicArthritis; very suitable for the comprehensive evaluation of Antivirals,Anti-inflammatory agents, and Vaccine candidates. | Mouse,NHPs |
| Sepsis/Septicemia Models | Sepsis, Septic Shock, Multiple OrganDysfunction Syndrome (MODS); ideally suited for the rigorous evaluation ofNovel Antibiotics, Anti-inflammatory/Immune-modulatory agents, Vasopressors. | Mouse,Rat, Rabbit, Pig |
| Infective Endocarditis Models | Infective Endocarditis (IE), Biofilmon heart valves; highly effective for the assessment of Antibiotics (e.g., high-dose,combinations) and Anti-biofilm agents. | Rabbit,Rat, Mouse |
| Bacteremia/Dissemination Models (Lyme Disease) | Lyme Disease (Borrelia burgdorferi)Acute and Chronic; specifically engineered for the evaluation of Antibiotics(e.g.,Doxycycline) and drugs targeting persistent forms, Vaccines. | Mouse,Dog, Guinea Pig |
| Rickettsiosis (Typhus) Models | Epidemic/Murine/Scrub Typhus; verysuitable for the comprehensive evaluation of Antibiotics (e.g., Doxycycline,Tetracycline) and Vaccines. | Mouse,Guinea Pig |
| Spirochetemia Models (Relapsing Fever) | Tick-Borne/Louse-Borne RelapsingFever; ideally suited for the rigorous evaluation of Antibiotics (e.g., Penicillin,Doxycycline) and management of Jarisch-Herxheimer Reaction. | Mouse,Rat, Hamster |
| Candidemia Models | Candidemia, Disseminated Candidiasis;highly effective for the assessment of Antifungal drugs (e.g.,Echinocandins, Azoles, Polyenes), Combination therapies. | Mouse,Rat, Rabbit |
| Invasive Aspergillosis (Systemic) Models | Disseminated Aspergillosis, InvasivePulmonary Aspergillosis (IPA); specifically engineered for the evaluation ofAntifungal drugs (e.g.,Azoles, Amphotericin B), Immunomodulatory agents. | Mouse,Rat, Rabbit |
| Disseminated Cryptococcosis (Systemic) Models | Cryptococcal Meningitis, DisseminatedCryptococcosis; very suitable for the comprehensive evaluation of Antifungaldrugs (e.g.,Amphotericin B, Flucytosine), Combination therapy. | Mouse,Rat, Guinea Pig |
| Plasmodium Parasitemia Models (Malaria) | Malaria (P. falciparum, P. vivax), CerebralMalaria; ideally suited for the rigorous evaluation of Antimalarials (e.g., Artemisinins,Chloroquine), Novel chemotherapeutics, Vaccine candidates. | Mouse,NHPs |
| American Trypanosomiasis Models (Chagas Disease) | Chagas Disease (Trypanosoma cruzi)(Acute and Chronic); specifically engineered for the evaluation ofAnti-protozoal drugs (e.g.,Benznidazole, Nifurtimox), Drugs for chronic cardiomyopathy. | Mouse,Guinea Pig, Dogs, Rat |
| Visceral Leishmaniasis Models | Visceral Leishmaniasis (VL),Kala-azar; very suitable for the comprehensive evaluation of Anti-leishmanialdrugs (e.g.,Amphotericin B, Miltefosine, Paromomycin), Immunomodulators. | Hamster,Mouse, Dog |
| Babesiosis Models | Human Babesiosis (Babesia spp.);ideally suited for the rigorous evaluation of Anti-protozoal combinations (e.g., Atovaquone +Azithromycin, Quinine + Clindamycin). | Mouse,Hamster, Mongolian Gerbil |
Measurements
Our primary evaluation of therapeutic success in viral models focuses on core indicators like viral load, inflammatory mediators, hematological parameters, and diseased organ pathology. To provide a more comprehensive and scientifically rigorous assessment, we also employ the following advanced, in-depth measurement methods, utilizing cutting-edge technologies:
- Clinical outcome indicators: Evaluating survival rates, tracking weight changes, and utilizing behavioral scores (e.g., activity levels, food intake).
- Microbiological indicators: Quantifying bacterial and viral loads in blood and tissue via techniques like colony-forming unit (CFU) counting and quantitative PCR.
- Immunological indicators: Measuring cytokines like IL-6 and TNF-α, inflammatory markers such as CRP and PCT by ELISA or MSD, and performing immune cell phenotyping through flow cytometry.
- Physiological and pathological indicators: Monitoring of body temperature, complete blood count (CBC), comprehensive assessment of coagulation dysfunction (e.g., D-dimer, PT/APTT), and examination of organ pathological sections using H&E and IHC.
- Support imaging technology: In vivo imaging (such as Bioluminescence Imaging, BLI) and fluorescent labeling techniques are used for real-time tracking of infection sites and drug distribution. PET-CT/Micro-CT can be utilized for damage assessment in specific organs (e.g., heart, bones).
Applications
- Disease Modeling: The models simulate complex clinical infection processes by reproducing pathogen dissemination, organ homing via the blood, and the subsequent lethal inflammatory responses like sepsis and viral hemorrhagic fever.
- Drug Discovery and Development: The models are essential tools for evaluating the in vivo efficacy of novel antimicrobial drugs, vaccines, and monoclonal antibodies, as well as for determining optimal PK/PD relationships and dosing regimens.
- Therapeutic Strategy Optimization: The models are used to refine combination and sequential treatment regimens for existing drugs and to validate the role of non-pharmacological interventions, such as immunomodulators and phage therapy, in mitigating organ damage and improving prognosis.
- Mechanism Study: The models elucidate the intricate pathogenesis of bloodstream infections by characterizing dynamic host-pathogen interactions under hemodynamic shear stress, revealing key processes such as endothelial barrier dysfunction, immune evasion strategies, and the dysregulated coagulation cascades that precipitate systemic failure.
Our Advantages
- Interdisciplinary Expertise: Our team comprises specialists in biology, pharmacology, and translational medicine, delivering comprehensive and professional technical support throughout the entire process of model design, experimental execution, and data interpretation.
- Diverse Pathogen Coverage: Our established models incorporate a variety of common pathogens, and we also possess the capability to develop models using client-provided, unique, or resistant pathogens.
- Advanced Detection Platform: We utilize fluorescent markers to modify pathogens, allowing for the visualization and tracking of the infection process in vivo. We also construct numerous circulatory system infection models to effectively replicate complex clinical challenges.
- Model Development Proficiency: We have established comprehensive models in various species, such as mice, rats, and Non-Human Primates (NHPs), covering diseases like sepsis, endocarditis, vasculitis, dengue fever, and malaria. These models are custom-tailored for drug pharmacology and pharmacodynamic research and development.
- Professional Technology & One-Stop Service: Leveraging our extensive experience and professional technology in preclinical research and development, we can efficiently complete preclinical efficacy verification and safety evaluation for clients. We offer an end-to-end, one-stop service, from model customization and data collection to final report delivery, thereby significantly accelerating project initiation and reducing the time costs associated with in-house model development.
- Biological Sample Management: We provide robust biological sample library management capabilities, supporting long-term sample storage and facilitating retrospective analysis and subsequent in-depth research.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: How do your models achieve high clinical relevance for Bloodstream Infection (BSI)/Sepsis?
A: Our models replicate human-like conditions by challenging the host with clinically relevant pathogens. We measure key outcomes like survival rate, bacterial burden in target organs, systemic cytokine storm, and critical organ dysfunction markers (e.g., lactate, transaminases). This allows for direct translation of PK/PD data to clinical settings.
-
Q: Which specialized in vivo models are utilized for pharmacodynamic assessment?
A: We primarily use the murine Cecal Ligation and Puncture (CLP) model, the gold standard for polymicrobial sepsis, alongside neutropenic and non-neutropenic intravenous challenge models. These platforms enable precise PK/PD target determination needed to achieve bacterial reduction and improve survival across various levels of host immunocompetence.
-
Q: Can your platform evaluate novel non-antibiotic therapies?
A: Yes. Beyond standard antibiotics, our models measure systemic host responses, including cytokine levels (IL-6, TNF-α) and markers of organ injury. This allows for the robust assessment of novel anti-inflammatory, immunomodulatory, and support therapies.
Published Data
DENV infection induces critical systemic damage in BALB/c mice, manifested by behavioral deficits (e.g., ruffled fur, paralysis) and severe hematological disorders (thrombocytopenia, vascular leakage). Crucially, a combination therapy utilizing Hematopoietic Stem Cells and Endothelial Progenitor Cells effectively reverses these pathologies, facilitating a step-by-step return to normal behavioral and blood indices.
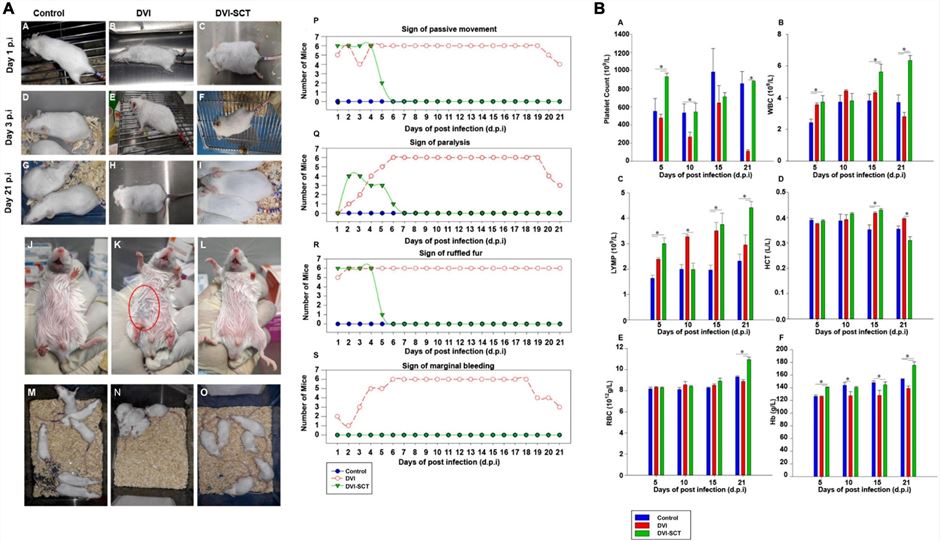 Fig.2 The effects of stem cell therapy on the behavioral status and blood parameters of dengue virus-infected mice.2,3
Fig.2 The effects of stem cell therapy on the behavioral status and blood parameters of dengue virus-infected mice.2,3
References
- Zhang, Hao et al. "Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis." Clinical and translational medicine vol. 13,1 (2023): e1170. https://doi.org/10.1002/ctm2.1170
- Sakinah, S et al. "Stem Cell Therapy in Dengue Virus-Infected BALB/C Mice Improves Hepatic Injury." Frontiers in cell and developmental biology vol. 9 637270. https://doi.org/10.3389/fcell.2021.637270
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
