Sensory System Infection Modeling & Pharmacodynamics Services
Introduction
Sensory system infectious diseases impact essential organs, including the eyes, ears, nose, and throat, and pose significant global health challenges. Annually, around 120 million cases of conjunctivitis are reported worldwide, with a cumulative incidence exceeding 80% among children under five. Diseases such as invasive fungal sinusitis carry a high mortality rate of 30-50%, often leading to severe and permanent functional impairment. Creative Biolabs leverages its extensive, multi-year experience in establishing infectious disease animal models and conducting pharmacological and efficacy evaluations. We offer a broad range of Sensory System Infection Models to meet diverse research needs. Through these preclinical animal models, we primarily support the pharmacological and efficacy studies of topical/local drugs, such as eye drops and ear drops, targeted biologics, and novel vaccines, accelerating the new drug development and commercialization process.
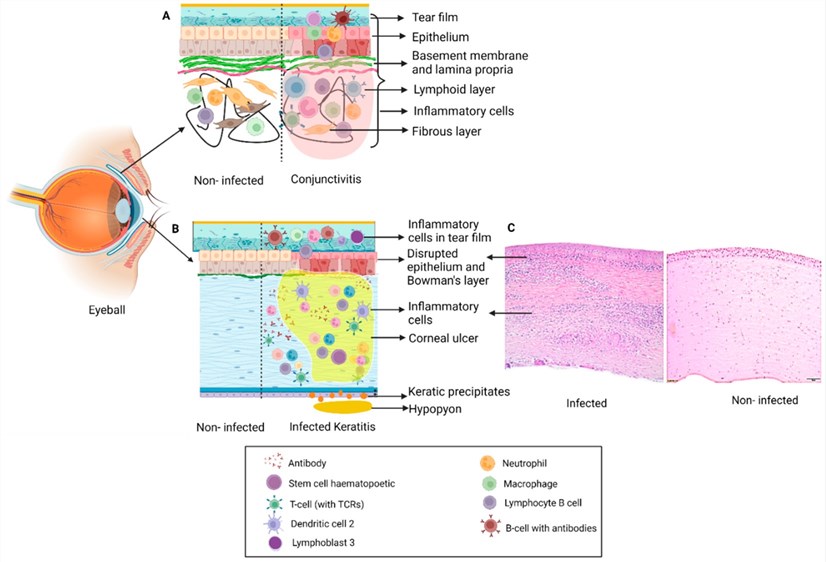 Fig.1 Schematic diagram of changes in ocular tissue infection.1,4
Fig.1 Schematic diagram of changes in ocular tissue infection.1,4
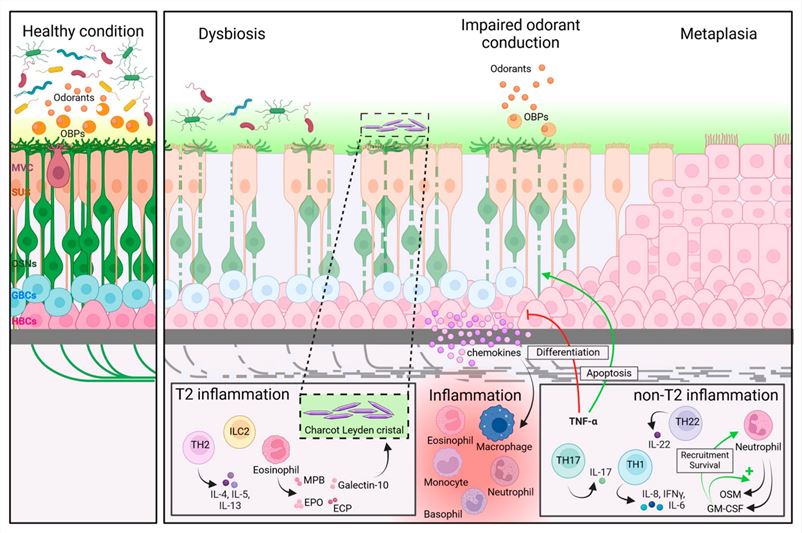 Fig.2 Possible disease mechanisms of olfactory dysfunction in chronic rhinosinusitis.2,4
Fig.2 Possible disease mechanisms of olfactory dysfunction in chronic rhinosinusitis.2,4
Available Sensory System Infection Models
Our advanced model platform provides comprehensive coverage for ophthalmic (eye), otic (ear), and nasopharyngeal (nose/throat) infections caused by a wide spectrum of pathogens, including bacteria, fungi, viruses, and parasites. These high-fidelity models are specifically engineered to address critical drug development challenges, generate clinically predictive data that guides formulation optimization, mitigates risk of permanent damage, and accelerates regulatory approval for topical/local drugs.
| Sensory System Infection Models | Related Disease & Drug Evaluation | Animal Species |
| Bacterial Keratitis Models | Bacterial Keratitis (e.g., Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae), Corneal Ulceration, Vision Loss; ideally suited for the definitive evaluation of Broad-spectrum and next-generation Topical Antibiotics (e.g., Fluoroquinolones, Aminoglycosides), Anti-biofilm agents, and Anti-inflammatory adjuncts. | Mouse, Rabbit |
| Simple Herpes Simplex Virus Keratitis Models | HSV Keratitis, Corneal Blindness, Dendritic Ulcers, Viral Latency; provides a powerful translational platform for the assessment of Next-generation Antivirals (e.g., Acyclovir, Ganciclovir), latency-reversing agents, and ocular Immunomodulators. | Rabbit, Mouse |
| Viral Conjunctivitis Models | Adenoviral Conjunctivitis (Pink Eye), Pharyngoconjunctival Fever; engineered for the reliable pre-clinical screening of Supportive care strategies, targeted Antivirals (e.g., Cidofovir), and anti-inflammatory agents. | Mouse, Guinea Pig, Rabbit, NHPs |
| Chlamydial Conjunctivitis Models | Inclusion Conjunctivitis (neonatal and adult), Genital Tract Infection; highly effective for the rigorous comparative evaluation of Antibiotic efficacy (e.g., Azithromycin, Doxycycline) and optimization of systemic delivery for ocular chlamydial eradication. | Mouse, Guinea Pig, Rabbit, NHPs |
| Gonococcal Conjunctivitis (Gonoblennorrhea) Models | Gonococcal Conjunctivitis (Severe, rapidly destructive), Neonatal Ophthalmia; instrumental for the critical assessment of Novel Antibiotics (to combat resistance) and efficacy of standardized prophylactic regimens. | Rabbit, Mouse |
| Blepharitis Models (Squamous, Ulcerative, Angular) | Inflammation of the Eyelid Margin (often Staphylococcus or Demodex); optimized for the systematic evaluation of Topical Antibiotics / Antiparasitics, advanced anti-inflammatory agents, and specialized tear film substitutes. | Mouse, Rat, Rabbit |
| Trachoma Models | Trachoma (Chronic follicular conjunctivitis leading to blindness); ideally suited for the definitive evaluation of Mass drug administration, Antibiotics (e.g., Azithromycin), advanced Vaccine candidates, and public health intervention strategies. | Mouse, Guinea pig, Rabbit, NHPs |
| Dacryocystitis Models | Infection of the Lacrimal Sac (often S. aureus, S. pneumoniae); provides a robust platform for the assessment of Optimal systemic Antibiotic penetration and strategies for pre/post-surgical management. | Rabbit, Dog |
| Uveitis Models | Infectious Uveitis (e.g., CMV, Toxoplasma, Mycobacterium), Ocular Inflammation; engineered for the reliable evaluation of Specific Antivirals / Antibiotics / Antifungals, targeted immunosuppressants, and corticosteroid efficacy. | Mouse, Rat, Guinea pig, Rabbit, NHPs |
| Intraocular Infection Models (General) | Endophthalmitis, Panophthalmitis (e.g., post-surgical bacterial or fungal infection); highly effective for the rigorous evaluation of Optimal drug concentrations (intravitreal and systemic) and novel adjuncts to surgical intervention (vitrectomy). | Mouse, Rat, Rabbit, NHPs |
| Onchocerciasis (River Blindness) Models | Onchocerciasis (Onchocerca volvulus), Chronic Dermatitis; instrumental for the critical assessment of New macrofilaricidal agents and strategies for eliminating the Wolbachia endosymbiont (e.g., Doxycycline). | Mouse, Gerbil, Cattle, NHPs |
| Otitis Externa Models | Otitis Externa (Swimmer's Ear, e.g., P. aeruginosa, S. aureus); optimized for the systematic evaluation of Efficacy and retention of topical Antibiotics / Antifungals, and delivery formulations. | Mouse, Rat, Guinea Pig, Rabbit, Dog |
| Otitis Media Models | Acute Otitis Media (AOM), Chronic Otitis Media (COM) (e.g., S. pneumoniae, H. influenzae); ideally suited for the definitive evaluation of Novel Antibiotics, next-generation Vaccines, and anti-biofilm therapeutic strategies. | Mouse, Rat, Rabbit, Chinchilla |
| Herpetic Ear Infection Models | Herpetic Otitis Externa / Media, Ramsay Hunt Syndrome (Varicella-Zoster Virus, VZV); provides a robust platform for the assessment of High-efficacy Antivirals (e.g., Acyclovir, Valacyclovir) and combination therapies with Corticosteroids. | Mouse, Rabbit, Guinea Pig |
Measurements
We offer a variety of highly advanced measurements for evaluating drug efficacy in Sensory System Infection Models, utilizing state-of-the-art technologies. These methods are designed to generate scientifically rigorous and clinically predictive data that assesses the effectiveness, including but not limited to:
- Clinical Observation and Scoring: Detailed recording of external disease signs (e.g., corneal opacity, ocular discharge, otorrhea) using standardized clinical scoring systems (e.g., corneal opacity score for Keratitis).
-
Visual/Auditory Function Tests: Objective assessment of functional impairment and recovery:
- Ophthalmic: Measurement of intraocular pressure (IOP), fundus imaging, and specialized visual acuity tests to assess the preservation of sight.
- Otic: Auditory Brainstem Response (ABR) and Distortion Product Otoacoustic Emissions (DPOAE) tests to precisely quantify hearing loss and evaluate the drug's protective effect against damage caused by infection or ototoxicity.
- Pathogen Load and Pharmacokinetics: Use of Digital Droplet PCR (ddPCR) or highly sensitive RT-qPCR to determine the absolute viral, bacterial, or fungal load in local samples, such as tear film, inner ear fluid (perilymph/endolymph), and nasopharyngeal washes. Auditory Brainstem Response (ABR) and Distortion Product Otoacoustic Emissions (DPOAE) tests to precisely quantify hearing loss and evaluate the drug's protective effect against damage caused by infection or ototoxicity.
- Pathogen Load and Pharmacokinetics: Detailed microscopic examination of critical tissues (e.g., cornea, retina, cochlea, nasopharyngeal epithelium) to assess inflammatory infiltration, tissue damage, and fibrotic changes. Use of specialized dyes (e.g., Evans Blue, FITC-dextran) to quantify the integrity of the blood-retina barrier or the tympanic membrane, providing evidence of infection-related damage and drug-mediated repair.
- Molecular Mechanism and Immunity: Multiplexed assays to quantify local inflammatory mediators (IL-6, TNF-α, chemokines) in tear film or inner ear fluid to analyze the drug's anti-inflammatory or immune-modulatory effects. Use of RT-qPCR or RNA sequencing to study the expression of immune and inflammatory genes in affected sensory tissues, providing deep insight into the disease mechanism and drug target verification.
Applications
- Disease Modeling: The models simulate the pathological processes of local infection, inflammatory damage within highly specialized tissues (e.g., eyes, ears, nose), and pathogen dissemination to the central nervous system.
- Mechanism Study: The models elucidate the distinct pathogenesis of ocular and otic infections by reconstructing pathogen transgression of the blood-retinal and blood-labyrinth barriers, decoding the molecular pathways that precipitate the collapse of local immune privilege, and characterizing the inflammatory fibrosis and cytotoxicity that result in irreversible damage to specialized sensory epithelia and permanent functional deficits.
- Drug Discovery and Development: The models serve as critical platforms for screening novel topical drugs, focusing on evaluating their local pharmacokinetics (Local PK), cross-barrier penetration efficiency, and the validation of advanced targeted delivery systems.
- Infection Treatment: The models are used to optimize combined local and systemic treatment regimens and to assess the potential ototoxicity and visual toxicity of drugs (like antibiotics) on sensitive sensory functions.
Our Advantages
- Advanced Technical Advisory Team and R&D Capabilities: Our team comprises experts in infectious diseases, veterinary medicine, statistical analysis, and biology, with cutting-edge technology and extensive experience in both animal modeling and drug efficacy testing for sensory system disease.
- Resource and Facility Advantages: Our state-of-the-art facilities boast advanced equipment such as in vivo imaging systems and high-throughput sequencing platforms. We maintain a diverse array of animal resources, including mice, rats, rabbits, and non-human primates, alongside a variety of established sensory system infection models with comprehensive technical parameters, facilitating swift project initiation.
- One-Stop Service: We offer a complete service cycle with customized models, including model establishment, indicator detection, data statistical analysis, and result interpretation, to accelerate the R&D process.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: Which areas do your Sensory System Infection Model services primarily focus on?
A: We specialize in using specific in vivo animal models to evaluate therapeutics for ocular (e.g., keratitis, endophthalmitis) and otolaryngological/ENT (e.g., otitis media, sinusitis) infections. We provide precise Pharmacodynamic (PD) assessment to accelerate drug development in these complex, localized infection sites.
-
Q: What is the scientific edge of your in vivo animal model platform?
A: Our platform includes diverse models, often utilizing specific species like rabbits for corneal/syphilis models and chinchillas for otitis media models. We precisely simulate the infection pathways (e.g., corneal scratch, transtympanic inoculation), ensuring high clinical pathological fidelity.
-
Q: Why are in vivo models critical for sensory system infection drug evaluation?
A: Sensory organs possess unique barrier structures and drug clearance mechanisms. In vivo models are essential for accurately assessing the drug's local PK, tissue penetration, and true efficacy within these complex microenvironments, which is unattainable in vitro.
-
Q: How do you assess drug efficacy in ocular infection models?
A: We employ a multi-modal assessment including clinical scoring (e.g., opacity, inflammation grade), microbial load quantification (cornea or vitreous humor), non-invasive in vivo imaging (e.g., OCT), and histopathological analysis, providing comprehensive macroscopic-to-microscopic efficacy data.
-
Q: Which key challenges and metrics do you focus on for ear/sinus infections?
A: We primarily address the challenge of achieving effective drug exposure in the middle ear cavity and nasal sinus mucosa. Assessment includes the degree of mucosal damage, ciliary function, microbial load in middle ear fluid, and inflammatory cytokine levels, guiding optimization of local delivery regimens.
-
Q: How does your service help address the local toxicity of drugs?
A: Sensory organs are highly sensitive to drug toxicity. Our in vivo models evaluate potential local toxicity to the retina, auditory hair cells, or vestibular function, alongside systemic safety. We conduct specialized assessments like electrophysiology (e.g., ERG) and audiology tests for fine-grained safety profiling.
-
Q: How does your service help shorten our drug development timeline?
A: By providing highly predictive in vivo efficacy data and a clear dose-response relationship, we help clients rapidly select the most promising candidates and optimize local concentration and frequency, thereby reducing the risk and cost of later-stage clinical trial failures.
-
Q: Can you handle non-standard routes of administration, such as intravitreal or intratympanic injections?
A: Yes. Our platform excels in these complex local administration techniques, including precise intravitreal, intratympanic injections, and various topical instillations/irrigations. This ensures that the drug's true clinical exposure conditions are replicated in vivo.
-
Q: What critical support does our collaboration provide for our IND submission?
A: Our pharmacodynamics reports serve as critical non-clinical supporting data for IND submission, especially in demonstrating the drug's efficacy and safety regarding sensitive tissue penetration and local clearance. The data we provide strengthens regulatory confidence in the drug's site-specific efficacy.
Published Data
Enterovirus A71 (EV-A71) infection model: Infection with EV-A71 in AG6 mice caused a 38% reduction in tear secretion and disrupted tear film stability, leading to a 21% decrease in lacrimal gland size with acinar vacuolization, corneal epithelial exfoliation, and a 20% loss of goblet cells, while elevated serum HMGB1 levels verified the infection-induced ocular dryness and structural defects in the lacrimal gland.
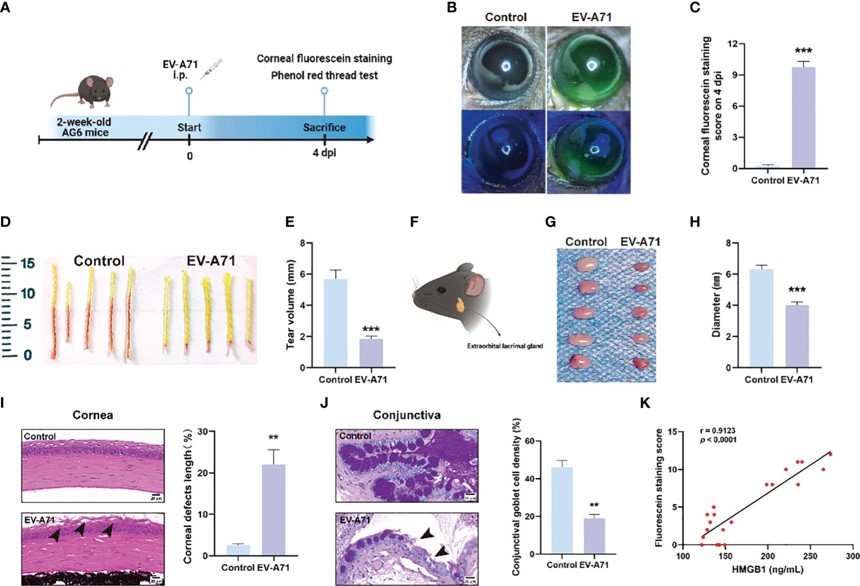 Fig. 3 EV-A71 induced reduction of tear volume and the abnormality of LGs.3,4
Fig. 3 EV-A71 induced reduction of tear volume and the abnormality of LGs.3,4
References
- Das, Samayitree et al. "Ocular Surface Infection Mediated Molecular Stress Responses: A Review." International Journal of molecular sciences vol. 23,6 3111. https://doi.org/10.3390/ijms23063111
- Dekeyser, Agnès et al. "Olfactory Loss in Rhinosinusitis: Mechanisms of Loss and Recovery." International journal of molecular sciences vol. 25,8 4460. https://doi.org/10.3390/ijms25084460
- Zhou, Nan et al. "Enterovirus A71 infection-induced dry eye-like symptoms by damaging the lacrimal glands." Frontiers in cellular and infection microbiology vol. 14 1340075. https://doi.org/10.3389/fcimb.2024.1340075
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
