Skin & Soft Tissue System Infection Modeling & Pharmacodynamics Services
Introduction
Skin and soft tissue infections (SSTIs), such as cellulitis, pyoderma, and necrotizing fasciitis, are common infectious diseases. SSTIs impose a significant global economic burden due to prolonged hospitalization and specialized wound care, and severe forms can lead to amputation, permanent functional loss, and sepsis. However, the incidence and mortality risk (e.g., extremely high mortality rate for necrotizing fasciitis) are significantly increased in severe and complex SSTIs caused by pathogens like methicillin-resistant Staphylococcus aureus (MRSA), recalcitrant biofilm infections, and necrotizing fasciitis. Creative Biolabs has developed diverse animal models that faithfully recapitulate the pathological process of various SSTIs. These high-fidelity models are essential translational tools, bridging the gap between in vitro discovery and clinical application by providing predictive in vivo efficacy data necessary for treating complex and life-threatening wound infections. These advanced models serve as critical in vivo tools for the pharmacological and efficacy evaluation of novel topical/systemic antibiotics, antifungals, antivirals, and wound healing therapies, thereby accelerating the entire preclinical development process.
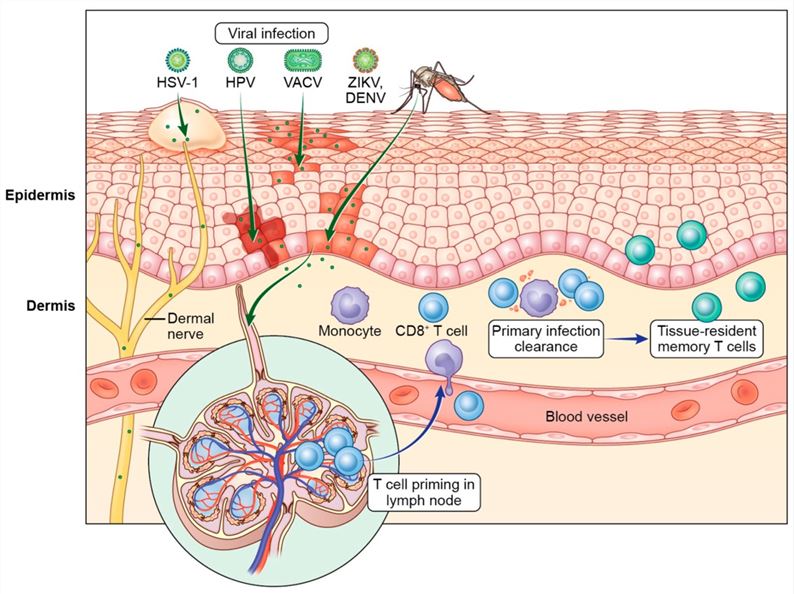 Fig1. T cell control of cutaneous viral infections. Viruses such as vaccinia virus (VACV), herpes simplex virus type 1 (HSV-1), Zika virus (ZIKV), dengue virus (DENV), and human papillomavirus (HPV) breach the skin barrier to establish infection in the epidermis and dermal immune cells.1
Fig1. T cell control of cutaneous viral infections. Viruses such as vaccinia virus (VACV), herpes simplex virus type 1 (HSV-1), Zika virus (ZIKV), dengue virus (DENV), and human papillomavirus (HPV) breach the skin barrier to establish infection in the epidermis and dermal immune cells.1
Available Skin and Soft Tissue System Infection Models
Our SSTI Modeling Platform offers comprehensive coverage across various infection sites, including subcutaneous, muscle/fascial, and burn infection models. These platforms are engineered to faithfully reproduce a wide spectrum of clinical pathologies, from herpes-like, tinea-like, warts, pustules, and papules to localized signs like redness, swelling, and pain. These models provide crucial support for the pharmacological and pharmacodynamic studies.
| Skin and Soft TissueSystem Infection Models | Related Disease& Drug Evaluation | Animal Species |
| Herpes Simplex Virus (HSV) SkinInfection Models | Oral/GenitalHerpes, Herpes Labialis (Cold Sores), Recurrent Lesions; ideally suited forthe definitive evaluation of Next-generation Antivirals (e.g., Acyclovir,Valacyclovir), novel Topical formulations, and Immunomodulators targetingrecurrence. | Mouse,Guinea Pig, Rabbit |
| Herpes Zoster (Shingles) InfectionModels | HerpesZoster, Postherpetic Neuralgia (PHN); provides a powerful translationalplatform for the assessment of High-efficacy Antivirals (e.g., Acyclovir,Famciclovir), Prophylactic/Therapeutic Vaccines (e.g., Live attenuated, Recombinant),and Analgesics for PHN management. | Mouse,Guinea Pig, NHPs |
| Varicella-Zoster (Skin) InfectionModels | Chickenpox(Varicella), Primary VZV infection; engineered for the reliable pre-clinicalscreening of Novel Antivirals and efficacy of Varicella Vaccines. | Mouse,Guinea Pig, NHPs |
| Verruca Vulgaris/Planar Wart InfectionModels | CommonWarts, Planar Warts (Human Papillomavirus, HPV); instrumental for the robustdevelopment of Antivirals, Immunomodulators (e.g., Imiquimod), and advanced chemical orimmunologic ablation agents. | Mouse,Rabbit |
| Coxsackievirus Myositis InfectionModels | Hand,Foot, and Mouth Disease (HFMD), Myositis, Viral Enanthem; very suitable forthe systematic evaluation of Targeted Antivirals and supportive carestrategies. | Mouse,NHPs |
| Impetigo Models | Impetigo(S. aureus or S. pyogenes),Contagious superficial infection; highly effective for the rigorousevaluation of Topical Antibiotics (e.g., Mupirocin, Retapamulin), SystemicAntibiotics (e.g.,Cephalexin), and Vaccine candidates. | Mouse,Rabbit |
| Folliculitis/Furuncle/Carbuncle Models | Folliculitis,Furuncles (Boils), Carbuncles (S. aureus); optimized for the accelerated assessment ofTopical/Systemic Antibiotics (especially against MRSA/drug-resistant strains) andnovel adjuncts to surgical drainage. | Mouse,Rat |
| Cellulitis And Erysipelas Models | Cellulitis,Erysipelas (S.pyogenes, S.aureus); provides a robust platform for the assessment ofPenetration and efficacy of Antibiotics (e.g., Penicillins, Cephalosporins) andAnti-inflammatory adjuncts. | Mouse,Rabbit |
| Necrotizing Fasciitis Infection Models | NecrotizingFasciitis (Polymicrobial, often S. pyogenes); ideally suited for the definitive evaluationof High-dose IV Antibiotic regimens (Clindamycin for toxin suppression),optimization of Surgical Debridement, and Immunoglobulin (IVIG) therapy. | Mouse,Rat |
| Gas Gangrene Infection Models(Clostridial Myonecrosis) | GasGangrene (C.perfringens), Rapidly destructive muscle infection; instrumentalfor the critical assessment of Antibiotic combinations (e.g., Penicillin,Clindamycin), Antitoxins, and Hyperbaric Oxygen Therapy protocols. | Mouse,Guinea Pig |
| Tinea Corporis/Cruris/Pedis Models | Ringworm,Athlete's Foot, Jock Itch (Dermatophytes); very suitable for the systematicevaluation of Topical Antifungal Drugs (e.g., Azoles, Terbinafine) and OralAntifungals (e.g.,Griseofulvin, Itraconazole). | GuineaPig, Mouse |
| Onychomycosis Models | NailFungus (Trichophytonspp.); optimized for the accelerated assessment of Oral AntifungalDrugs (e.g.,Terbinafine, Itraconazole) and Novel topical delivery systems (e.g., lacquers) fornail penetration. | GuineaPig, Mouse |
| Cutaneous Candidiasis Models | SkinCandidiasis, Intertrigo (C. albicans); highly effective for the rigorous evaluationof Topical Antifungal Drugs (e.g., Azoles, Nystatin) and Systemic Antifungals (forsevere cases). | Mouse,Rat, Rabbit |
| Sporotrichosis Infection Models | Sporotrichosis(Rose Gardener's Disease, Sporothrix spp.); engineered for the reliable pre-clinicalscreening of Systemic Antifungal Drugs (e.g., Itraconazole, Amphotericin B) andthermo-therapy adjuncts. | Mouse,Cat |
| Scabies Infection Models | Scabies(Sarcoptes scabiei),Intense pruritus; instrumental for the critical assessment of Acaricides (e.g., Permethrin,Ivermectin, Lindane) and combination therapy regimens to combat resistance. | Rabbits,Pig, Dog |
| Pediculosis (Lice) Infection Models | Pediculosis(Head/Body/Pubic Lice); very suitable for the systematic evaluation ofPediculicides (e.g.,Permethrin, Malathion, Ivermectin) and molecular studies on drug resistance. | Mouse,Rabbit |
| Cutaneous Leishmaniasis InfectionModels | CutaneousLeishmaniasis (CL), Skin Ulcers (Leishmania spp.); ideally suited for thedefinitive evaluation of Antiprotozoal Drugs (e.g., Miltefosine, Antimonials,Amphotericin B) and next-generation Vaccines. | Mouse,Hamster, Dog |
| Cutaneous Larva Migrans (CLM) Models | CreepingEruption (Hookworm larvae migration); provides a robust platform for theassessment of Antihelminthic Drugs (e.g., Albendazole, Ivermectin) and efficacyof topical therapy. | Mouse,Guinea Pig |
| Trichinellosis Models | Trichinellosis(Trichinella spiralis),Larval migration/myositis; engineered for the critical evaluation ofAntihelminthic Drugs (e.g.,Albendazole, Mebendazole) and Corticosteroids for inflammation management. | Mouse,Rat, Pig |
| Leprosy (Hansen's Disease) InfectionModels | Leprosy(Mycobacterium leprae),Tuberculoid/Lepromatous forms; instrumental for the specialized assessment ofMulti-Drug Therapy (MDT) (e.g., Dapsone, Rifampicin, Clofazimine) and Vaccinecandidates. | Mouse,NHPs, Zebrafish |
| Diabetic Foot Models | DiabeticFoot Ulcers, Polymicrobial Infection, Osteomyelitis; optimized for theaccelerated assessment of Broad-spectrum Antibiotics, Growth factors/Woundhealing agents, and optimization of surgical intervention protocols. | Mouse,Rat |
| Subcutaneous Infection Models | Abscesses,localized deep skin infection; highly effective for the rigorous evaluationof Antibiotics (often against MRSA), Drainage techniques, and Anti-biofilm agents. | Mouse,Rat |
| Muscle/Fascial Infection Models | Pyomyositis,Deep space infection, provides a robust platform for the assessment ofAntibiotic penetration into deep tissue and optimization of surgicalintervention. | Mouse,Rat |
| Burn Infection Models | BurnWound Sepsis, Skin/Soft Tissue Infection; ideally suited for the definitiveevaluation of Topical and systemic Antibiotics, Silver-based dressings, andAnti-sepsis agents. | Mouse,Rat, Guinea Pig, Pig |
Measurements
Creative Biolabs utilizes cutting-edge technology and multi-dimensional evaluation techniques for a comprehensive analysis of disease mechanisms and drug efficacy. to ensure the scientific rigor & reproducibility of results and guarantee the highest quality of service, these methods encompass, but are not limited to:
- Clinical Observation and Scoring: These assessments include evaluating the general condition, body temperature, weight fluctuations, and mobility, alongside specific skin manifestations (e.g., ulcers, redness, and eschar formation).
- Microbiological and Hematological Tests: These analyses focus on determining bacterial colonization and proliferation, drug resistance, and viral load. Additionally, whole blood analysis and blood biochemistry are performed to identify inflammatory markers and evaluate the model's severity.
- Histopathological Analysis: H&E staining is used to assess inflammatory cell infiltration, the degree of tissue damage, and pathogen localization. Meanwhile, special stains such as Gram/PAS are utilized to identify pathogens. Masson's Trichrome staining is employed to evaluate collagen deposition and the degree of fibrosis in models of chronic infection and wound healing.
- Molecular Biology and Immunological Tests: These methods include detecting cytokines through ELISA or cytokine microarray, and performing mechanistic investigations using real-time fluorescence quantitative PCR (qPCR) or Western blotting (WB).
- Imaging Tests: Micro-CT is primarily used for the assessment of bone or calcification (e.g., diabetic foot complicated by osteomyelitis).
Applications
- Disease Modeling: The models simulate the pathological processes of pathogen invasion through the skin barrier, the formation of localized infections like abscesses or necrotizing fasciitis, and the development of highly resistant biofilms in chronic wounds.
- Mechanism Study: The models elucidate the distinct pathogenesis of cutaneous and soft tissue infections by reproducing pathogen invasion across stratified epidermal and dermal architectures, decoding the mechanisms of biofilm-mediated antimicrobial resistance and toxin-driven tissue necrosis, and characterizing the dysregulated wound healing processes that precipitate abscess formation, cellulitis, and lethal necrotizing fasciitis.
- Drug Discovery and Development: The models serve as critical platforms for screening novel systemic and topical agents, focusing on evaluating drug penetration through skin and necrotic tissue, bioavailability, and in vivo microbicidal efficacy.
- Infection Treatment: The models are used to optimize combination therapies and surgical interventions for resistant and highly virulent strains, while assessing the efficacy of novel dressings and anti-biofilm strategies.
Our Advantages
- Technological and Model Development Strengths: We offer a comprehensive range of pathogens, including bacterial, fungal, viral, and mixed infections, across multiple animal species such as mice, rats, rabbits, and pigs.
- Interdisciplinary Team and Proven Expertise: Our team comprises experts in microbiology, pathology, pharmacology, and related fields. They possess extensive experience in establishing SSTIs models, as well as in the early-stage development of drugs, vaccines, medical devices, and related interventions.
- Comprehensive Customized Service: We possess the ability to devise comprehensive customized service and solutions for complex scientific challenges, to meet diverse client needs. We strictly adhere to confidentiality agreements, follow standardized operating procedures (SOPs), ensure data traceability and internal quality control, and provide detailed analysis and visualization reports.
- One-stop services: We also specialize in model development for other disease areas, and offer one-stop services encompassing target validation, pharmacokinetics, and toxicology.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What major drug development challenges do your SSTI Model Services address?
A: We focus on using high-translational in vivo animal models to evaluate the efficacy of novel anti-infective drugs for complex SSTIs (e.g., cellulitis, abscesses, burn wound infections, diabetic foot infections). Our core value is providing precise local Pharmacokinetic/Pharmacodynamic (PK/PD) data.
-
Q: How does your in vivo model platform ensure scientific accuracy?
A: We have established various models, including mouse and rat muscle infection models, subcutaneous abscess models, full-thickness skin excision models, and immunocompromised models. We can simulate infections from Gram-positive, Gram-negative bacteria, and fungi, ensuring high fidelity to human clinical infections pathologically and microbiologically.
-
Q: What key metrics do you use to evaluate the efficacy of SSTI therapeutics?
A: We use multi-dimensional quantitative assessment, including microbial load quantification at the infection site (bacteria/fungal/viral titers), lesion size/healing rate measurement, histopathological analysis (inflammation and necrosis severity), and inflammatory biomarker levels, ensuring comprehensive and reliable data.
-
Q: How do your models demonstrate value when treating resistant infections?
A: Our models can be customized to incorporate Multi-Drug Resistant (MDR) organisms, particularly Methicillin-Resistant Staphylococcus aureus (MRSA). We evaluate not only the drug's bactericidal activity but also its ability to penetrate the abscess wall and necrotic tissue, guiding optimal dosing regimens for hard-to-treat infections.
-
Q: How do you assess the efficacy and safety of drugs delivered via topical routes?
A: For topical agents (e.g., ointments, gels, dressings), we precisely measure the drug's local PK in the stratum corneum and subcutaneous tissue. This is integrated with an assessment of skin irritation (dermatitis) and wound healing speed to optimize the local delivery system and dosing frequency.
-
Q: How does your service help accelerate the drug transition from the preclinical stage to IND?
A: We provide clear PK/PD relationships and dosing regimen recommendations. This data directly supports First-in-Human (FIH) dose selection. By confirming drug efficacy in complex SSTI models, we significantly reduce the risk of clinical trial failure due to insufficient efficacy.
-
Q: Can your platform support the assessment of drugs for complex/chronic wound infections?
A: Yes. We have experience establishing chronic/complex wound models (e.g., diabetic foot ulcer infections in relevant models) and integrating Biofilm infection assessment. This is highly commercially valuable for developing therapeutics aimed at treating persistent infections and eliminating biofilms.
Published Data
In the mouse SSTI primary infection-vaccination-secondary infection model, α-hemolysin (Hla) expressed during Staphylococcus aureus infection inhibits the protective efficacy of subsequent vaccines. Specifically, the protective inhibition of the Hla vaccine is antigen-specific and related to the Hla expression in the primary infection. It reduces the expansion of vaccine-induced IL-17A and IFNγ effector T cells, while the antibody response is not affected.
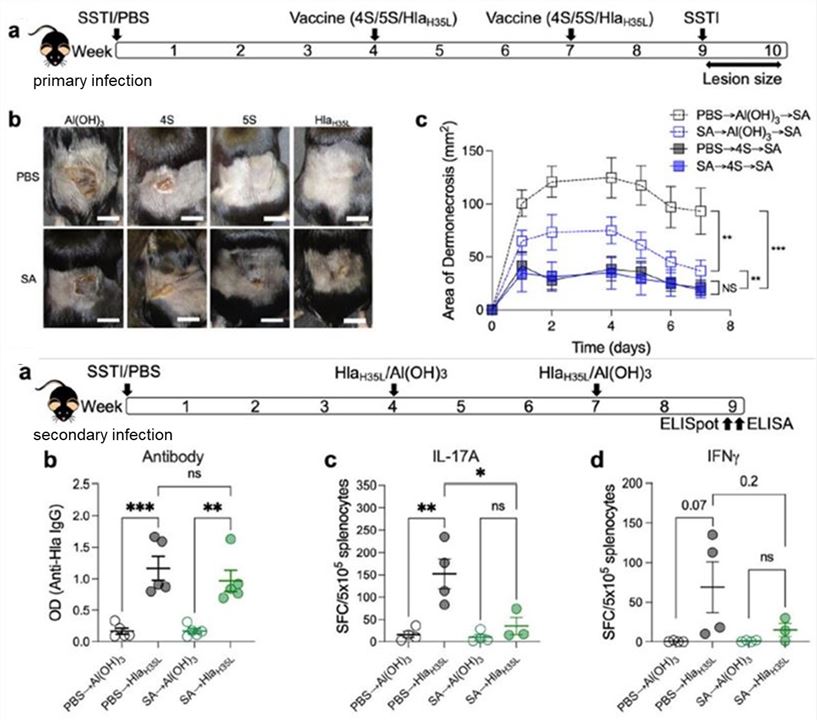 Fig.2 Mouse SSTI primary infection-vaccination-secondary infection model.2
Fig.2 Mouse SSTI primary infection-vaccination-secondary infection model.2
References
- Pei, Luxin, and Heather D Hickman. "T Cell Surveillance during Cutaneous Viral Infections." Viruses vol. 16,5 679. https://doi.org/10.3390/v16050679. Distributed under Open Access license CC BY 4.0, without modification.
- Teymournejad, Omid et al. "Toxin expression during Staphylococcus aureus infection imprints host immunity to inhibit vaccine efficacy." NPJ vaccines vol. 8,1 3. https://doi.org/10.1038/s41541-022-00598-3. Distributed under Open Access license CC BY 4.0, with modification.
For Research Use Only.
