Zoonotic Disease Infection Modeling & Pharmacodynamics Services
Introduction
Zoonotic diseases, including those caused by the Ebola virus, rabies virus, and Zika virus, are naturally transmissible between humans and animals. The incidence and transmission of these diseases are increasing significantly, affecting millions globally and causing hundreds of thousands of deaths annually, thereby posing a severe public health challenge. To address this, Creative Biolabs has developed highly effective non-rodent, rodent, and non-human primate (NHP) animal models for zoonotic diseases and possesses the capability to conduct studies in high-level biosafety laboratories. These models are dedicated to investigating disease pathogenesis, evaluating pharmacology, and accelerating the R&D process for key therapeutics, including broad-spectrum antivirals, monoclonal antibodies, and novel vaccines, such as mRNA and adenovirus vectors.
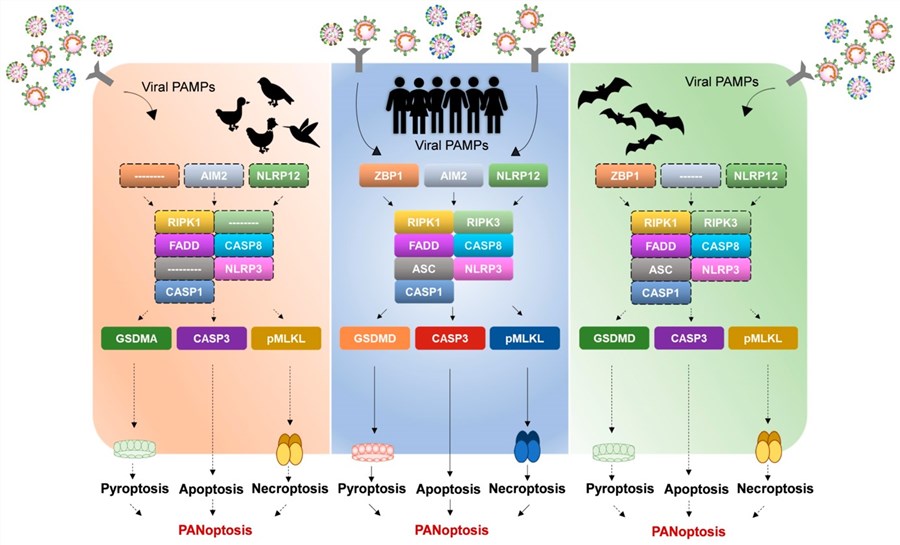 Fig.1 Altered PANoptosomes in birds and bats indicate differential PANoptosis regulation and immune tolerance of zoonotic viruses.1
Fig.1 Altered PANoptosomes in birds and bats indicate differential PANoptosis regulation and immune tolerance of zoonotic viruses.1
Available Zoonotic Diseases Infection Models
We offer models across various species, including mice, rats, hamsters, guinea pigs, and highly translatable non-human primates (NHPs). The zoonotic disease models currently offered by Creative Biolabs include:
| Zoonotic Diseases Infection Models | Related Disease | Relevant Drug Evaluation | Animal Species |
| Ebola Virus (EBOV)Infection Models | Ebola Virus Disease (EVD), ViralHemorrhagic Fever (VHF) | Antivirals (e.g., Remdesivir),Monoclonal Antibodies (e.g.,Inmazeb, Ebanga), Vaccines. | NHPs, Mouse, Guinea Pig |
| Severe Fever withThrombocytopenia Syndrome (SFTS) Virus Models | SFTS, Haemorrhagic Fever,Thrombocytopenia | Antivirals (e.g., Ribavirin,Favipiravir), Supportive care, Monoclonal Antibodies. | Mouse, Hamster, Ferret |
| Monkeypox VirusModels | Monkeypox (MPOX), Smallpox(Poxviruses) | Antivirals (e.g., Tecovirimat,Cidofovir), Vaccines (e.g.,ACAM2000, JYNNEOS). | Mouse, Cotton Rat, NHPs |
| Rabies Virus Models | Rabies, Fatal Encephalitis | Post-Exposure Prophylaxis (PEP) (Vaccine and Rabies Immune Globulin,RIG), Novel antivirals targeting CNS penetration. | Mouse, Rat, Hamster |
| Avian Influenza Virus(AIV) Models | Avian Flu (e.g.,H5N1, H7N9), Pandemic potential, Viral Pneumonia | Antivirals (e.g.,Neuraminidase inhibitors, Cap-dependent endonuclease inhibitors), Vaccines,Combination therapy. | Mouse, Ferret, Chicken, Duck |
| West Nile Virus (WNV)Models | West Nile Fever, Encephalitis, Meningitis | Antivirals (Experimental, e.g., Nucleoside analogs), Vaccines, Immunomodulatoryagents. | Mouse, Hamster |
| Brucellosis Models | Brucellosis (B.melitensis, B.abortus), Undulant Fever, Chronic infection | Antibiotics (e.g.,Doxycycline + Rifampicin/Gentamicin), Novel antibiotics targetingintracellular survival, Vaccines (Animal use). | Mouse, Guinea Pig, Cattle, Goat |
| Plague Models | Plague (Yersiniapestis), Pneumonic Plague, Bubonic Plague | Antibiotics (e.g.,Streptomycin, Gentamicin, Fluoroquinolones), Vaccines (Experimental). | Mouse, Guinea Pig, Rabbit |
| Lyme Disease Models | Lyme Disease (Borreliaburgdorferi), Arthritis, Carditis, Neuroborreliosis | Antibiotics (e.g.,Doxycycline, Amoxicillin), Drugs targeting persistent forms (persisters),Vaccines. | Mouse, Dog, NHPs |
| Leptospirosis Models | Leptospirosis (Leptospira spp.),Weil's Disease (renal/hepatic failure) | Antibiotics (e.g.,Doxycycline, Penicillin G), Supportive care, Vaccines (Animal use). | Hamster, Guinea Pig, Mouse |
Measurements
Creative Biolabs selects customized assessment metrics based on pathogen, species, and ethical standards. We leverage cutting-edge technologies like ddPCR, LC-MS/MS, and MSI to guarantee the highest quality efficacy data. The detection methods and indicators for pharmacodynamic studies, including but not limited to:
- Clinical Endpoints & Survival Analysis: Clinical Symptom Observation: We use validated scoring systems to monitor disease progression (e.g., body weight, neurological score, fever profile) and survival as the primary endpoint.
- Systemic & Serological Biomarker Analysis: By collecting blood, tissue, cerebrospinal fluid (CSF), and secretion samples, we perform pathogen nucleic acid detection (PCR), serological antibody level determination (ELISA), and blood biochemical analysis. Special emphasis is placed on cytokine/chemokine profiling and coagulation function indices (PT/APTT/D-dimer) to assess systemic inflammatory response and organ damage.
- Pathogen Load & Clearance Kinetics: Absolute quantification of viral/bacterial load (ddPCR/qPCR) in systemic and target organs (e.g., spleen, lymph nodes).
- Histopathological Analysis: We section and stain tissues from infected animals to observe histopathological changes and assess the drug effects on lesioned tissues.
- PK/Tissue Distribution Mapping: Use of LC-MS/MS/MSI to precisely map drug concentration in blood, CSF, and deep tissue foci, crucial for CNS-targeting agents.
Applications
- Disease Modeling: The models simulate the complex process of cross-species spillover from animal reservoirs to humans, model vector-borne disease dynamics, and assess pathogen virulence and transmission potential in human hosts.
- Mechanistic Research: The models are core tools for elucidating the molecular mechanisms that determine pathogen host range and cross-species barrier function, as well as for studying virulence factors and latent infection ecology.
- Drug Discovery and Development: The models are critical platforms for rapidly screening broad-spectrum antivirals, vaccines, and monoclonal antibodies against emerging or high-risk pathogens (like Ebola), focusing on vaccine protective efficacy and systemic drug distribution.
- Infection Treatment: The models are used to optimize treatment regimens for rare and complex zoonotic diseases and to evaluate the impact of drugs or vaccines on pathogen load in animal hosts to block transmission to humans.
Our Advantages
- Interdisciplinary Expertise & Depth: We bring together an interdisciplinary team of talent, including senior R&D scientists, veterinary experts, microbiologists, and statistical analysts. Our team members deeply understand zoonotic pathological mechanisms and are proficient in the construction of various animal models and efficacy testing methods, ensuring the scientific rigor of experimental design and data interpretation.
- High-Containment Platform & Advanced Technology: We are equipped with international first-class experimental facilities and detection equipment, and have established a comprehensive biosafety laboratory system. This infrastructure supports experiments involving highly pathogenic microorganisms, guaranteeing data accuracy and operational safety.
- Standardized Efficiency & Timely Delivery: We implement Standardized Operating Procedures (SOPs) and a rigorous project management system. This systematic delivery model efficiently advances research projects, significantly shortening project cycles and gaining valuable time for our clients' new drug development.
- Customized & End-to-End Solutions: Guided by client needs, we offer comprehensive services from experimental protocol design to data delivery. We can flexibly customize experimental plans based on research objectives and budget, meeting diverse needs and ensuring your R&D investment achieves the best commercial return.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What unique advantages does your platform offer in model construction?
A: Our model advantage lies in high translatability and diversity. We construct a model spectrum including non-human primates (NHP), ferrets, and various rodent species. These models accurately simulate the pathogen's natural infection routes and host-specific pathophysiology, thereby providing highly predictive in vivo data for human diseases.
-
Q: Why are these models indispensable for evaluating the efficacy of zoonotic disease therapeutics?
A: Zoonotic pathogens often possess unique capabilities for invasion and intracellular replication, frequently leading to systemic, lethal diseases. In vivo models are the only tools that can comprehensively evaluate systemic drug distribution, tissue targeting, and intracellular activity. This is crucial for the rapid translation of key therapeutics, such as broad-spectrum antivirals, monoclonal antibodies, and novel vaccines, targeting emerging/re-emerging threats.
-
Q: How does your pharmacodynamic assessment system quantify efficacy for lethal diseases and ensure scientific rigor?
A: We use survival rate/mortality as the primary endpoint, combined with multi-dimensional quantitative analysis: quantifying pathogen load across multiple organs; performing histopathological analysis; and monitoring key biomarkers (e.g., cytokines). Furthermore, the models support staged intervention assessments for post-exposure prophylaxis (PEP) and disease progression phases, guiding precise clinical strategies.
-
Q: How do these models data provide commercial value and a competitive advantage to clients?
A: Our data grants clients a unique commercial head start. We provide rapid, reliable in vivo efficacy data against high-risk pathogens, accelerating the drug development process. By quickly validating drug effectiveness, we help clients gain a significant first-to-market advantage in the field of special pathogens.
Published Data
The mouse mpox virus model successfully demonstrated the protective efficacy of the vaccine candidate. Secondary immunization significantly enhanced the titers of vaccinia virus-neutralizing antibodies, and these antibodies exhibited potent direct viral neutralization activity. Crucially, post-challenge analysis showed no evidence of inflammation or pathological damage in critical organs, including the heart, liver, spleen, lungs, and kidneys, confirming both systemic safety and organ protection mediated by the induced immune response.
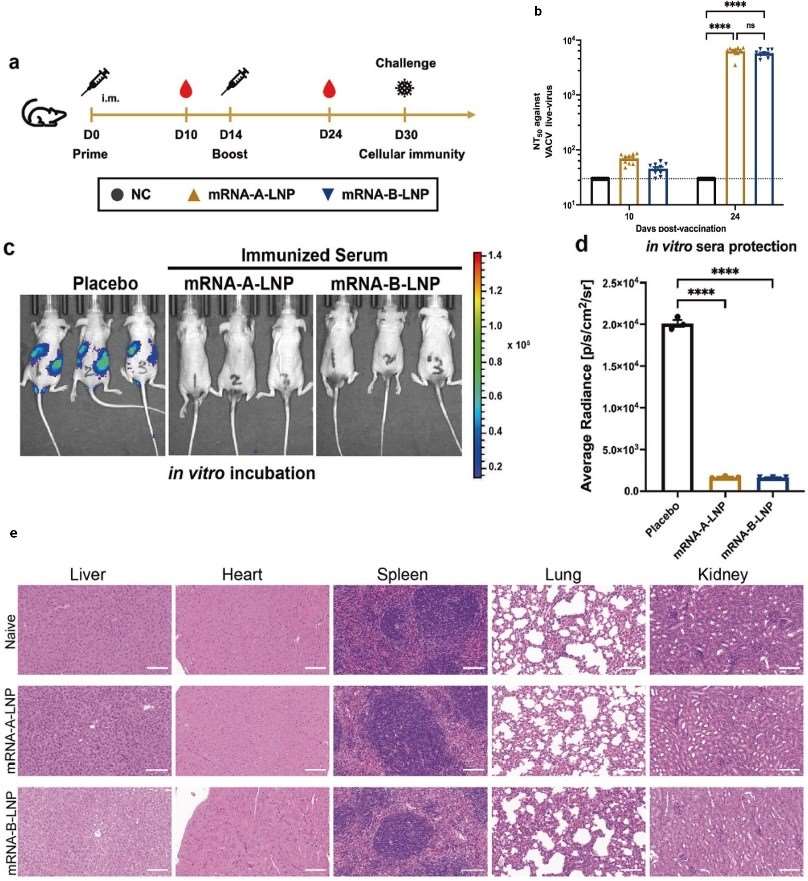 Fig. 2 The quadrivalent mRNA vaccine against mpox virus induces an immune response.2
Fig. 2 The quadrivalent mRNA vaccine against mpox virus induces an immune response.2
References
- Chandra, Anantika, and Sannula Kesavardhana. "PANoptosis Regulation in Reservoir Hosts of Zoonotic Viruses." Viruses vol. 16,11 1733. https://doi.org/10.3390/v16111733. Distributed under Open Access license CC BY 4.0, without modification.
- Sang, Ye et al. "Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus." Signal transduction and targeted therapy vol. 8,1 172. https://doi.org/10.1038/s41392-023-01432-5. Distributed under Open Access license CC BY 4.0, with modification.
For Research Use Only.
