Respiratory System Infection Modeling & Pharmacodynamics Services
Introduction
Respiratory infectious diseases, such as influenza, pneumonia, tuberculosis (TB), COVID-19, Middle East Respiratory Syndrome (MERS), Q Fever, respiratory syncytial virus (RSV) infection, and rhinovirus (HRV) infection, are major contributors to global morbidity and mortality. These diseases collectively account for millions of hospitalizations and are the leading infectious cause of death worldwide. Creative Biolabs utilizes a range of crucial animal models, including rodents, non-rodents, and non-human primates (NHPs), to replicate disease pathology, especially acute respiratory distress syndrome (ARDS) and chronic pulmonary injury. High-fidelity respiratory infection models are an indispensable tool for drug discovery, serving as the bridge between in vitro success and clinical application by providing predictive in vivo efficacy data. Our models comprehensively support the pharmacological and efficacy studies of small molecule drugs, inhaled formulations, anti-inflammatory/anti-fibrotic biologics, and vaccines, accelerating the new drug development and successful regulatory submissions.
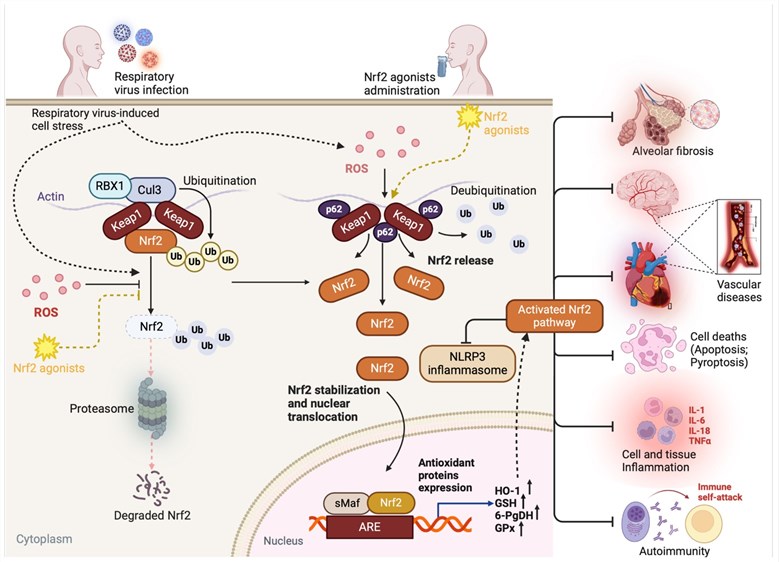 Fig.1 The role of the Nrf2 pathway in airway injuries due to viral respiratory infections. Viral respiratory infections are one of the leading causes of airway damage.1,3
Fig.1 The role of the Nrf2 pathway in airway injuries due to viral respiratory infections. Viral respiratory infections are one of the leading causes of airway damage.1,3
Available Respiratory System Infection Models
Creative Biolabs offers a comprehensive selection of thoroughly validated models induced by a range of pathogens, with detailed descriptions available below for a comprehensive understanding of the various respiratory infection models offered.
| Respiratory System Infection Models | Related Disease & Drug Evaluation | Animal Species |
| Influenza Virus Infection Models | Seasonal and Pandemic Influenza, Viral Pneumonia, ARDS; specifically engineered for the evaluation of Antivirals (e.g., Oseltamivir, Baloxavir), Vaccine candidates, and Immunomodulators. | Mouse, Ferret, Guinea pig, NHPs |
| Seasonal Coronavirus Infection Models | Common Cold, Mild Acute Respiratory Illness (ARIs); very suitable for the assessment of Supportive care, Antivirals (e.g., broad-spectrum), and Vaccines. | Mouse, Rat, Hamster |
| COVID-19 Infection Models | COVID-19 (Mild to Severe ARDS), Systemic Disease; highly effective for the rigorous evaluation of Antivirals (e.g., Paxlovid, Remdesivir), Vaccines (e.g., mRNA, viral vector), Monoclonal Antibodies, and Immunomodulators. | Mouse, Ferret, Hamster, NHPs |
| Combined Infection Models | Viral-Bacterial co-infections (e.g., post-flu bacterial pneumonia); ideally suited for the comprehensive evaluation of Combination Antibiotic and Antiviral therapy, and Adjunctive immunomodulators. | Mouse, Ferret, NHPs |
| Middle East Respiratory Syndrome (MERS-CoV) Infection Models | MERS, Severe Pneumonia, ARDS; specifically engineered for the evaluation of Antivirals (e.g., Ribavirin, Interferon), Vaccines, and Monoclonal Antibodies. | Mouse, Hamster, Camelus dromedarius, NHPs |
| Rhinovirus Infection Models | Common Cold, Asthma Exacerbation; very suitable for the assessment of Supportive care, Antivirals, and Anti-inflammatory drugs (for asthma/COPD exacerbations). | Mouse, Ferret, NHPs |
| Parainfluenza Virus Infection Models | Croup, Bronchiolitis, Pneumonia (especially in children); highly effective for the rigorous evaluation of Supportive care, Antivirals, and Vaccines. | Mouse, Ferret, Hamster, Guinea pig, NHPs |
| Respiratory Syncytial Virus (RSV) Infection Models | Bronchiolitis, Pneumonia (severe in infants/elderly); ideally suited for the comprehensive evaluation of Antivirals (e.g., Ribavirin), Monoclonal Antibodies (e.g., Palivizumab, Nirsevimab), and Vaccines. | Cotton Rat, Rat, Mouse, NHPs |
| Adenovirus Infection Models | ARIs, Viral Pneumonia, Pharyngitis; specifically engineered for the evaluation of Supportive care, Antivirals (e.g., Cidofovir in immunocompromised), and Vaccines. | Mouse, Rat, Ferret, Guinea pig, Rabbit, NHPs |
| Bacterial and fungal pneumonia Models | Pneumonia (e.g., S. pneumoniae, K. pneumoniae), Invasive Aspergillosis; very suitable for the assessment of Antibiotics (novel and combination therapies), Antifungals, and Vaccines. | Mouse, Rat, Rabbit |
| Mul-timicrobial (Viral-Bacterial-Fungal) Respiratory Tract Infection Models | Complex Aspiration Pneumonia, Severe Community/Hospital Acquired Pneumonia; highly effective for the rigorous evaluation of the combination of Antibiotics, Antivirals, Antifungals, and Immune modulators. | Mouse, Rat, Ferret, NHPs |
| Diphtheria Infection Models | Diphtheria, Diphtheritic Pharyngitis/Croup; ideally suited for the comprehensive evaluation of Antitoxin, Antibiotics (e.g., Penicillin, Erythromycin), and Vaccines (Toxoid). | Mouse, Hamster, Guinea Pig, Rabbit |
| Pneumonia Infection Models | Pneumonia (e.g., General, S. pneumoniae, H. influenzae, M. pneumoniae), Empyema; specifically engineered for the evaluation of Antibiotics (to achieve high lung concentration), and Vaccines. | Mouse, Rat, Rabbit, Ferret, NHPs |
| Tuberculosis Infection Models | Tuberculosis (Latent and Active), Drug-Resistant TB (MDR/XDR); very suitable for the assessment of Anti-Tuberculosis Drugs (e.g., Rifampicin, Isoniazid), Novel drug regimens, and Vaccines. | Mouse, Rat, Guinea Pig, Rabbit, NHPs |
| Pertussis Infection Models | Pertussis (Whooping Cough), Severe Respiratory Disease; highly effective for the rigorous evaluation of Antibiotics (e.g., Macrolides), Vaccines (e.g., aP/wP), and Antitoxin therapies. | Mouse, Rat, Guinea pig, Hamster, NHPs |
| Q Fever Infection Models | Q Fever, Atypical Pneumonia, Chronic Endocarditis; ideally suited for the comprehensive evaluation of Antibiotics (e.g., Doxycycline, Hydroxychloroquine combination for chronic), and Vaccines. | Mouse, Guinea Pig, Hamster, Sheep, NHPs |
| Mumps/Epidemic Parotitis Infection Models | Mumps, Parotitis, Orchitis, Meningitis; specifically engineered for the evaluation of Supportive care, Antivirals, and Vaccines (MMR). | Mouse, Cotton rat, Ferret, Hamster, NHPs |
| Epidemic Cerebrospinal Meningitis Infection Models | Meningitis/Septicemia caused by N. meningitidis (often starts as nasopharyngeal colonization); very suitable for the assessment of Antibiotics (e.g., Penicillin G, Ceftriaxone), Vaccines, and Adjunctive corticosteroids. | Mouse, Rat, Rabbit, Guinea pig, NHPs |
Measurements
Our Respiratory Infection Model measurements integrate a diverse array of advanced detection technologies and indicators. This integrated approach moves beyond basic viral load to deliver scientifically rigorous, mechanistically rich data, allowing for deeper insights into drug action and superior preclinical support, including but not limited to:
- Physiological and Functional Assessment: Monitoring of body weight, body temperature, and activity levels; conducting pulmonary function tests (e.g., respiratory rate, tidal volume, measurement of lung compliance/airway resistance); and assessing ARDS scoring.
- Pathogen Load & Molecular Analysis: RT-qPCR/qPCR is used to quantify the pathogen load in lung tissue, BALF (Bronchoalveolar Lavage Fluid), and blood; in situ hybridization is utilized for pathogen localization.
- Pathology & Imaging: H&E staining is used to evaluate pulmonary tissue inflammation, alveolar edema, and fibrosis; CT/X-ray is used to detect pulmonary consolidation; and in vivo imaging (BLI/fluorescent labeling) is utilized for real-time tracking of infection spread.
Applications
- Disease Modeling: The models simulate the full infection pathway, from aerosol transmission and respiratory colonization to acute lung injury and chronic biofilm formation, to study pathogen spread and virulence.
- Mechanism Study: The models elucidate the specific pathogenesis of airway infections by reproducing the physiological dynamics and mucociliary clearance, decoding the molecular basis of pathogen tropism and invasion, and characterizing the immune-mediated alveolar barrier disruption that culminates in acute lung injury (ALI) and respiratory distress syndrome (ARDS).
- Drug Discovery: They are essential for screening novel drugs and vaccines, with a special focus on evaluating the local pulmonary pharmacokinetics (PK) and the optimized delivery and effectiveness of inhaled formulations.
- Infection Treatment: The models guide the optimization of therapy by testing antibiotic combinations against Multidrug-Resistant (MDR) pathogens and assessing adjuvant strategies like Host-Directed Therapy (HDT) or phage therapy to reduce inflammation and improve recovery.
Our Advantages
- Frontier Technology and Expertise: Our team integrates multidisciplinary professionals in biology, medicine, and statistics, bringing extensive project experience to bear on complex challenges.
- Resource and Facility Advantages: We are equipped with professional aerosol generators and inhalation exposure systems, comprehensive pulmonary function testing instruments, and a mature library of animal models.
- Efficiency and Cost Advantages: Our standard operating procedures and extensive project management experience efficiently advance research projects, mitigating the risk of experimental failure due to inexperience and thereby reducing overall R&D costs.
- Data and Analysis Strengths: A stringent quality control system ensures data accuracy and reliability. Furthermore, our professional data analysis team utilizes advanced tools to explore and interpret complex datasets.
- Dynamic Communication and Feedback: Project progress is regularly synchronized via phone or email. We commit to responding to issues (e.g., anomalous experimental data) within 24 hours, providing actionable solutions for client decision-making.
- Flexible Adjustments and Responsive Support: In response to client inquiries (e.g., concerning cost optimization or cycle reduction), we provide alternative solutions (e.g., modifying animal model types or adjusting test indicator priorities) and update solution content in real time.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What is the core value proposition of your Respiratory Infection Model services?
A: Our core value is the precise and efficient assessment and optimization of novel anti-infective agents' efficacy, dosing regimens, and safety margins using clinically relevant in vivo animal models integrated with advanced PK/PD modeling. This significantly accelerates the translation of drugs from preclinical to clinical stages.
-
Q: What types of infections can the in vivo animal model platform simulate?
A: We offer customized models for various respiratory pathogens (e.g., Influenza, Pneumococcus, Tuberculosis). We utilize species, such as mice, ferrets, and NHPs, to simulate key pathological features in humans, including ARDS, pulmonary fibrosis, and mixed viral/bacterial infections, ensuring high translatability.
-
Q: How do you ensure the scientific reliability of in vivo efficacy data?
A: We employ a multi-dimensional assessment approach. Beyond measuring microbial burden in infected tissues, we incorporate histopathology, inflammation biomarker quantification, and other endpoints to systematically validate the drug's activity and disease mitigation effects, providing comprehensive mechanistic evidence.
-
Q: What support do your models offer against antibiotic resistance?
A: Our platform can assess drug activity against MDR strains, determine the Post-Antibiotic Effect (PAE), and help identify the risk of resistance emergence. This data directly informs the development of competitive combination and dosing strategies.
-
Q: Can you evaluate specialized delivery methods, such as inhaled formulations?
A: Yes. Our platform is equipped with professional inhalation delivery systems to accurately simulate clinical administration, allowing us to acquire local PK and efficacy data under realistic conditions. This is crucial for developing inhaled anti-infectives, resulting in data with high clinical translational value.
Published Data
After intratracheal inoculation with SARS-CoV-2, knock-in transgenic mice expressing hACE2 successfully established a human-like acute respiratory distress syndrome (ARDS) model. This model fully recapitulates the pulmonary pathological characteristics observed in severe COVID-19 patients, providing direct in vivo experimental evidence for elucidating the mechanisms of SARS-CoV-2-induced severe pneumonia and for evaluating targeted interventions, such as anti-ARDS drugs and vaccines.
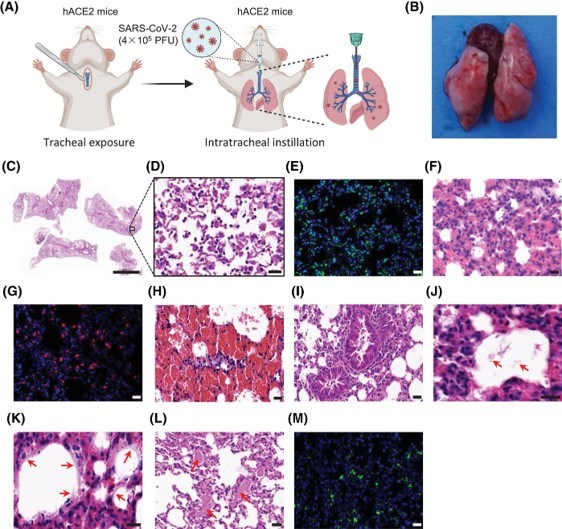 Fig.2 The pulmonary features of a mouse model for SARS-CoV-2-induced ARDS.2,3
Fig.2 The pulmonary features of a mouse model for SARS-CoV-2-induced ARDS.2,3
References
- Kombe Kombe, Arnaud John et al. "The Role of the Nrf2 Pathway in Airway Tissue Damage Due to Viral Respiratory Infections." International Journal of Molecular Sciences vol. 25,13 7042. https://doi.org/10.3390/ijms25137042
- Bi, Zhenfei et al. "Animal models for SARS-CoV-2 infection and pathology." MedComm vol. 2,4 548-568. https://doi.org/10.1002/mco2.98
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
