FcγRI Binding Assay Service
Creative Biolabs offers a specialized FcγRI binding assay to address the challenges of long drug development cycles, difficulties in antibody development, and the complex nature of clinical trials in the biopharmaceutical field. This essential service helps you accelerate drug discovery, develop highly specific antibodies, and streamline preclinical assessment through advanced high-throughput screening platforms and innovative protein engineering techniques.
FcγRI: Immune Role and Therapeutic Significance
The high-affinity Fc gamma receptor I (FcγRI, also known as CD64) is a pivotal player in the innate and adaptive immune systems, predominantly expressed on myeloid cells such as monocytes, macrophages, and neutrophils. It uniquely binds monomeric IgG with high affinity, initiating critical immune responses like antibody-dependent cellular cytotoxicity (ADCC) and the efficient clearance of immune complexes. In the current landscape of antibody therapeutic development, understanding these interactions is paramount. Genetic variations in FcγRs can significantly influence therapeutic outcomes, making precise characterization of FcγRI binding essential for optimizing antibody design, predicting in vivo efficacy, and ensuring drug safety. This deep understanding is crucial for overcoming development hurdles and advancing novel therapeutic candidates.
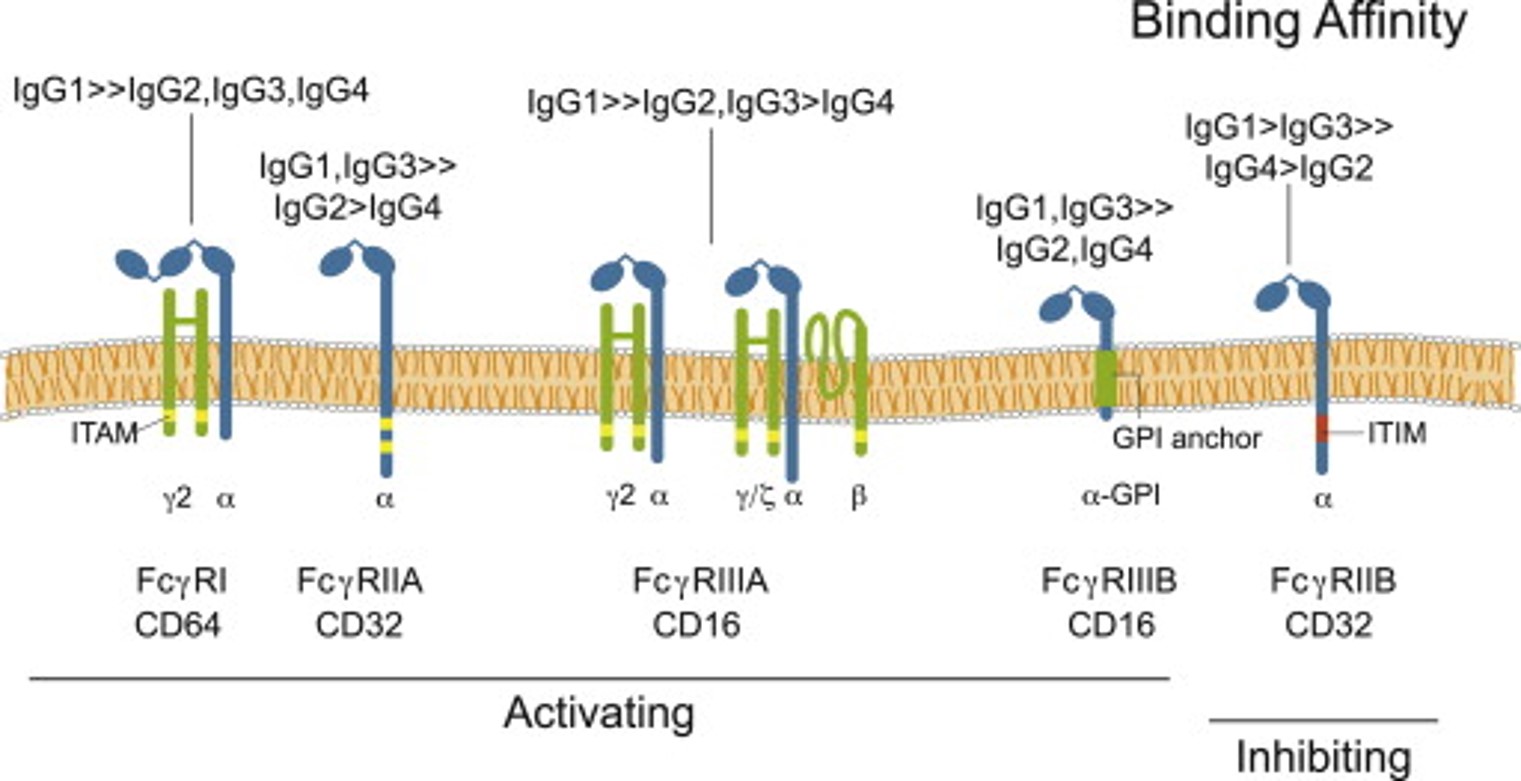 Fig.1 Fc receptors may have distinct Ig specificities.1
Fig.1 Fc receptors may have distinct Ig specificities.1
Our FcγRI Binding Assay
In the challenging landscape of biopharmaceutical development, where long drug development cycles and the demand for highly specific antibody therapeutics are constant pressures, Creative Biolabs' FcγRI binding assay offers a crucial solution. Our service is meticulously designed to provide precise and comprehensive data on the interaction between your antibody therapeutics and FcγRI. We follow rigorous principles, employing state-of-the-art platforms to delineate binding kinetics (association and dissociation rates) and affinity constants (KD). This is indispensable for rational antibody engineering and accurately predicting potential Fc-mediated effector functions.
Clients gain actionable insights to optimize antibody candidates, enhance their therapeutic index, and streamline the path to clinical development. The typical timeframe for these in-depth analyses ranges from 4 to 8 weeks, depending on project complexity. We uphold the highest standards of quality through rigorous experimental controls, advanced instrumentation, and expert data interpretation, guaranteeing reliable and reproducible results for every project.
Required Starting Materials
- Antibody or Fc-fusion protein samples: Highly purified therapeutic antibody or Fc-fusion protein candidates (typically >90% purity) in sufficient quantities (e.g., milligrams) for concentration series and replicates.
- Target Antigen Information: Any relevant information about the antibody's target antigen, including its sequence, known epitopes, and the intended therapeutic application, to help contextualize results.
- Detailed Project Objectives: A clear outline of your research questions, desired kinetic parameters, specific IgG subclasses or variants to be evaluated, and any unique assay conditions or comparability requirements.
Workflow
Step1. Initial Consultation & Design: Our scientific team partners with you to define project goals, then designs and optimizes the most relevant assay methodology to ensure maximum data quality.
Step2. Recombinant FcγRI Preparation: We meticulously prepare, purify, and validate high-quality, biologically active recombinant human FcγRI (CD64) protein in-house, guaranteeing consistent and reliable reagents for every study.
Step3. Assay Execution & Data Acquisition: Experienced scientists execute the selected assay on state-of-the-art analytical platforms. For SPR, FcγRI is immobilized, and antibody samples are flowed over at various concentrations to capture real-time association and dissociation events, generating raw sensorgram data.
Step4. Data Processing & Quality Control: Raw data undergoes rigorous processing, including baseline subtraction, blank subtraction, and normalization, followed by comprehensive quality control checks to validate data reliability and integrity.
Step5. Kinetic & Affinity Analysis: Specialized software models the processed data to yield crucial binding parameters: association rate (ka), dissociation rate (kd), and the equilibrium dissociation constant (KD), providing a quantitative measure of binding strength.
Selective Approaches at Creative Biolabs
We provide a variety of selective design approaches to meet the unique needs of each customer's project:
- ELISA
- Flow cytometry
- Surface plasmon resonance / Bio-layer interferometry: real-time, label-free full kinetic analysis.
Service Highlights
- High-Resolution Kinetic & Affinity Data: Obtain precise data crucial for selecting and optimizing antibody candidates with desired binding characteristics.
- Robust & Validated Platforms: Benefit from consistent and reproducible results, backed by our state-of-the-art technology and rigorous quality control.
- Rapid Turnaround Times: Keep your projects on schedule with our efficient workflows and commitment to timely data delivery.
- Deeper Understanding of Effector Functions: Gain detailed insights into how your antibody interacts with FcγRI, translating to a clearer understanding of its potential effector functions.
- Reduced Preclinical & Clinical Risk: Minimize risks in later development stages by accurately characterizing FcγRI binding early on, optimizing your therapeutic's profile for safety and efficacy.
Case Study
| Objective: | To measure the binding affinity of sample-1 with FcγRI/CD64 via the SPR method. | ||||||||||||||||||||||
| Assay Format: | Binding and fitting curves between samples with FcγRI/CD64: 1:1 binding model; | ||||||||||||||||||||||
| Results: | The fitting curves for Sample-1 binding to CD64 are shown in Fig.2. Using the 1:1 binding model, the captured CD64 can bind Sample-1 with an affinity constant of 2.99 x 10-9 M. | ||||||||||||||||||||||
|
Table 1 SPR result summary between sample-1 with FcγRI/CD64 |
|||||||||||||||||||||||
Customer Reviews
- [Unmatched Specificity Insights] [1 Month], A***a Patel
Creative Biolabs' FcγRI Binding Assay facilitated a direct comparison of our antibody candidates against both FcγRI and other Fc receptors. This provided clear insights into the desired specificity, helping us avoid potential off-target binding issues. The comprehensive data report was instrumental in guiding our antibody engineering efforts, allowing us to select the most promising candidates.
- [Reliable Data, Every Time] [3 Weeks], Prof. M***e Leen
We relied on Creative Biolabs for our Fc-fusion protein characterization, and their FcγRI Binding Assay delivered consistently reliable and reproducible data. Their robust experimental design and thorough data analysis addressed our concerns regarding potential batch variability and provided strong evidence for our regulatory submissions. The scientific support was exceptional.
FAQs
-
Q1: What is the primary role of FcγRI in the immune system, and why is its binding relevant to therapeutic antibodies?
A1: FcγRI, a high-affinity receptor for IgG, plays a crucial role in activating myeloid cells, mediating antibody-dependent cellular cytotoxicity (ADCC), and clearing immune complexes. For therapeutic antibodies, understanding FcγRI binding is critical because it dictates how effectively an antibody can engage immune effector cells to eliminate target cells, influencing both efficacy and potential off-target effects.
-
Q2: What techniques does Creative Biolabs utilize for its FcγRI Binding Assay, and which is most recommended?
A2: Creative Biolabs employs various state-of-the-art techniques, including ELISA, Flow Cytometry, and Surface Plasmon Resonance (SPR) or Bio-layer Interferometry (BLI). While all methods offer valuable insights, SPR/BLI are highly recommended for their ability to provide real-time, label-free kinetic and affinity data, offering a more comprehensive understanding of the interaction.
-
Q3: Can your FcγRI Binding Assay provide insights into how glycosylation affects antibody binding?
A3: Yes, our advanced platforms can be leveraged to investigate the impact of different glycosylation patterns on FcγRI binding. By testing glycoengineered antibody variants or using specific enzymatic treatments, we can help you understand how Fc glycan modifications influence binding affinity and kinetics, which is crucial for optimizing antibody function.
Related Sections
Creative Biolabs offers a comprehensive suite of complementary services to support every stage of your antibody therapeutic development, ensuring a holistic understanding of your molecule's characteristics and potential:
- FcγRIIa Binding Assay: Characterize binding to FcγRIIa (CD32a), another crucial activating receptor involved in ADCP and other myeloid cell functions.
- FcγRIIb Binding Assay: Investigate interactions with the only inhibitory Fc receptor, FcγRIIb (CD32b), critical for understanding immune regulation and potential off-target effects.
- FcγRIIIa Binding Assay: Essential for evaluating antibody-dependent cellular cytotoxicity (ADCC) mediated by NK cells, with a focus on clinically relevant polymorphisms.
- FcRn Binding Assay: Optimize pharmacokinetic properties, extend serum half-life, and guide dosing strategies by characterizing interactions with the neonatal Fc receptor.
- ADCC/ADCP Assays: Complement your binding data with direct cellular readouts to confirm and quantify the biological activity of your antibody.
Work with Creative Biolabs
Creative Biolabs is your premier partner for advanced biopharmaceutical research, delivering comprehensive FcγRI Binding Assay services that provide unparalleled insights into antibody-receptor interactions. Our extensive expertise, cutting-edge platforms, and unwavering commitment to quality ensure you receive the most reliable data to accelerate your drug discovery and development. Accelerate your therapeutic development with our global FcγRI Binding Assay services. Our expert team is ready to help you evaluate your therapeutics; contact us today to get started.
Reference
- Maverakis, Emanual et al. "Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review." Journal of autoimmunity vol. 57 (2015): 1-13. doi:10.1016/j.jaut.2014.12.002. Distributed under an Open Access License CC BY 4.0, without modification.
For Research Use Only.

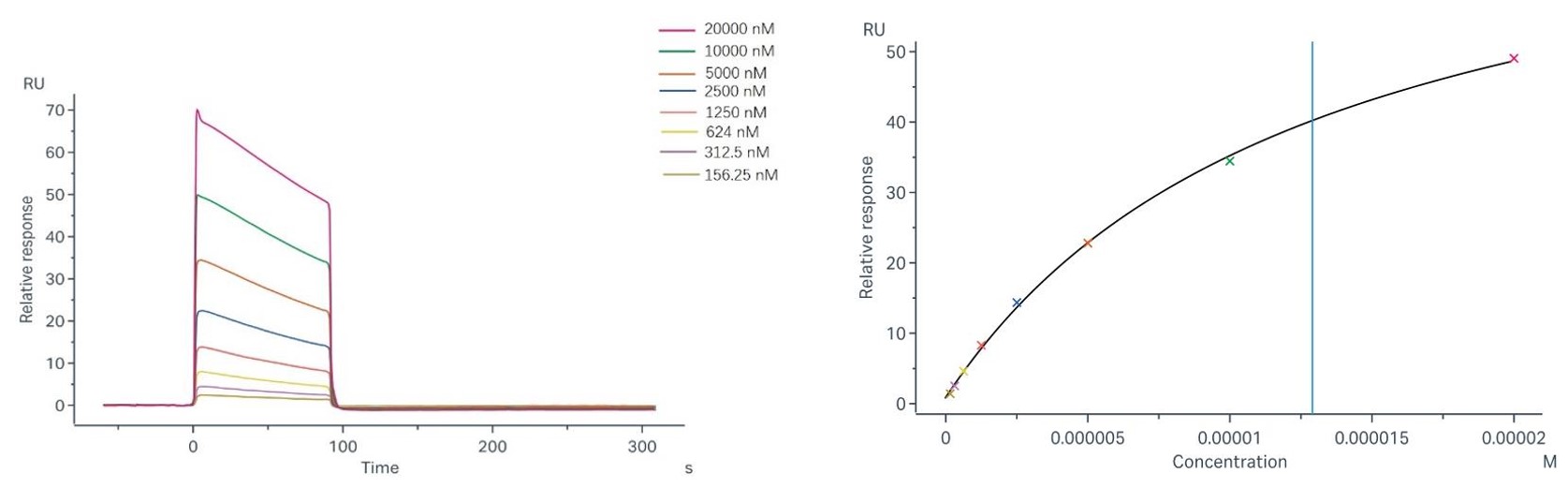 Fig.2 Binding and fitting curves between sample-1 with FcγRI/CD64 at different concentrations.
Fig.2 Binding and fitting curves between sample-1 with FcγRI/CD64 at different concentrations.