FcγRIIb Binding Assay
Creative Biolabs has been working on the development of correct assays to help our clients understand the properties and functions of their candidate monoclonal antibody (mAb)-based therapeutics. Especially, we have the capacity of providing a full range of FcγR binding assays with the highest quality and the most competitive price for global clients.
Introduction to FcγRIIb & Its Function
The Fc-γ receptor IIb (FcγRIIb), also known as CD32b, is the only inhibitory Fc receptor with low-affinity for Immunoglobulin G. FcγRIIb is a type I transmembrane receptor mainly expressed by immune cells, including B cells, dendritic cells, activated neutrophils, macrophages, basophils, and mast cells. In human, FcγRIIb presents a series of inhibitory activities in immunologic processes, such as (i) decrease antibody production and thus inhibits humoral immunity when expressed on B cells, (ii) prevent inappropriate maturation and immunogenic antigen presentation when expressed on dendritic cells, and (iii) inhibit other functions of FcγRs activation, like FcγR-mediated phagocytosis, the release of cytokines, and other pro-inflammatory mediators when expressed on other immune cells. Among these, FcγRIIb is particularly important in inhibiting B cell receptor-mediated activation and maintaining B cell tolerance since FcγRIIb is the principal FcγR expressed on B cells.
Mutations in the FCGR2B gene or abnormity of FcγRIIBs in humans can be associated with different infectious and autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, Goodpasture’s disease, idiopathic thrombocytopenic purpura, and so forth. Moreover, increasing evidence has indicated that antibodies that specifically bind FcγRIIb are useful in treating B cell and plasma cell malignancies.
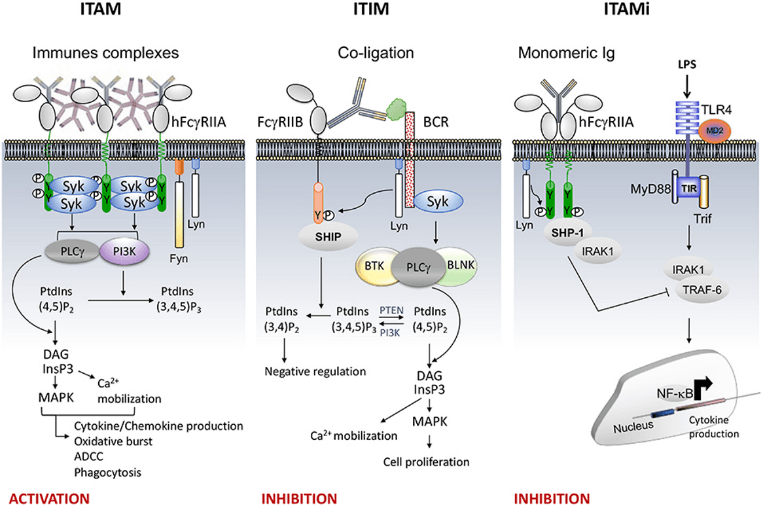 Fig.1 FcR signaling (e.g., FcγRII).1, 2
Fig.1 FcR signaling (e.g., FcγRII).1, 2
Our FcγRIIb Binding Assay
Creative Biolabs provides specialized analytical services for FcγRIIb binding characterization, utilizing multiple biochemical platforms to quantify receptor-ligand interactions and evaluate structural determinants such as glycosylation patterns. The workflow begins with a collaborative consultation phase to optimize assay parameters for precise interrogation of therapeutic antibody binding kinetics. High-throughput instrumentation and validated protocols yield quantitative binding metrics, structural analyses, and mechanistic interpretations of modulatory factors, presented in standardized technical reports. Technical experts oversee experimental design and data interpretation, ensuring scientifically robust deliverables to accelerate the development of antibody therapies targeting this immunoregulatory receptor.
Available Approaches
We provide a range of tailored methodologies to effectively address the design needs of our international clients' projects. Comprising, among other elements:
- ELISA
- Flow cytometry
- Surface plasmon resonance/ Bio-layer interferometry (recommended): real-time, label-free full kinetic analysis
Case Study
Case Study
- Objective: the study aims to measure the binding affinity of sample-1 with FcγRIIb/CD32b via the SPR method.
-
Assay Format:
Binding and fitting curves between sample with FcγRIIb/CD32b: steady-state model; - Results
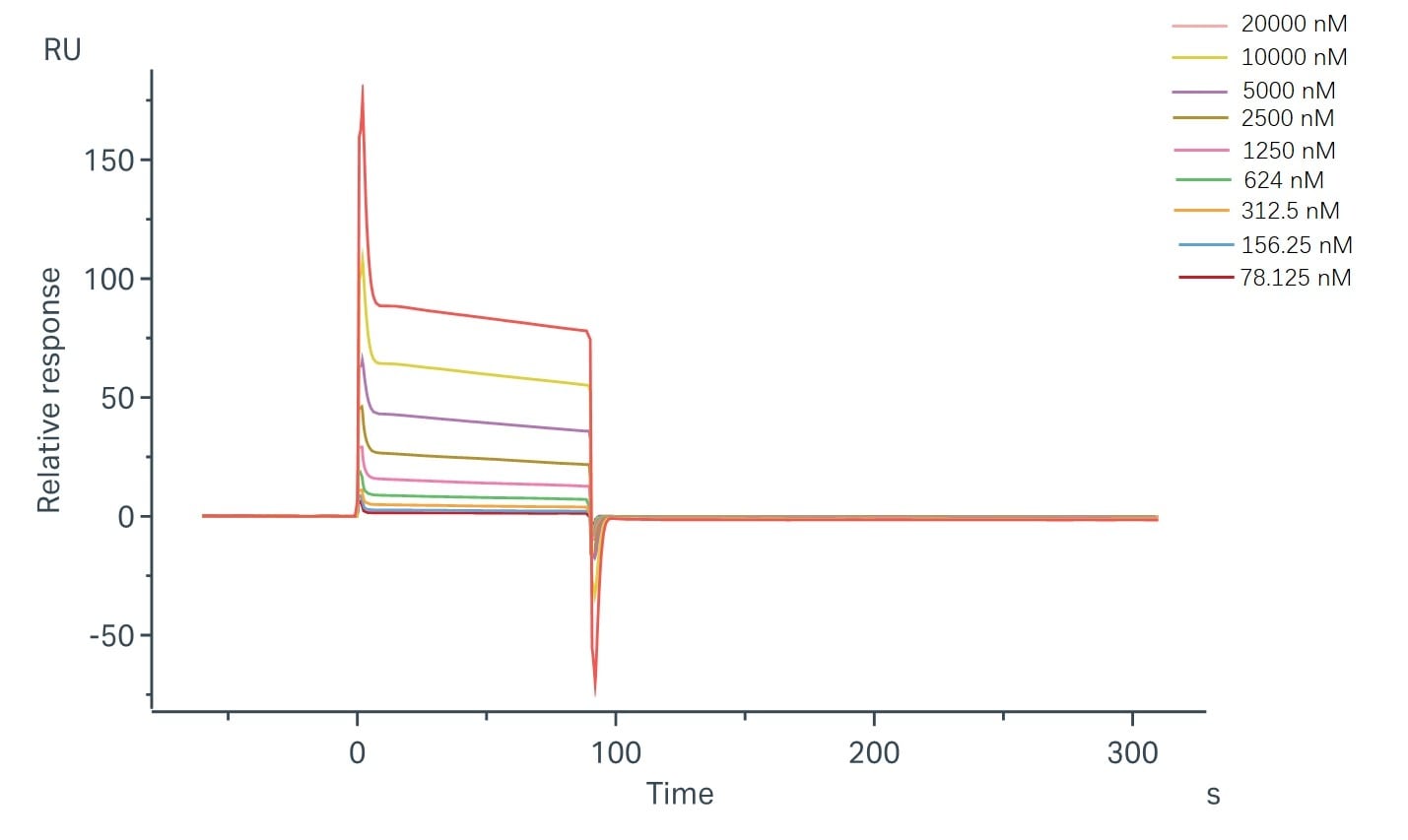
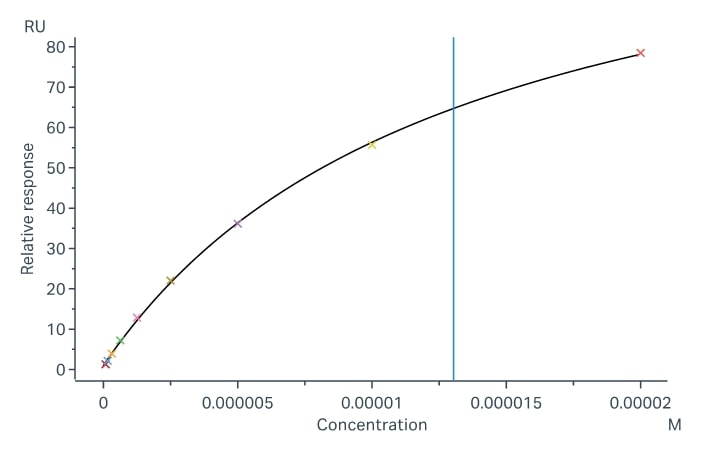 Fig.2 Binding and fitting curves between sample-1 with FcγRIIb/CD32b at different concentrations. (Creative Biolabs)
Fig.2 Binding and fitting curves between sample-1 with FcγRIIb/CD32b at different concentrations. (Creative Biolabs)
Table.1 SPR result summary between sample-1 with FcγRIIb/CD32b. (Creative Biolabs)
| Method | Ligand | Capture Level (RU) | Analyte | Analyte Conc. | ka (1/Ms) | kd (1/s) | KD (M) | Rmax (RU) | Chi² (RU²) | Fit method |
| His Capture | Human FcγRIIb/CD32b | 43.2 | Sample-1 | 78.125-20000 nM | NA | NA | 1.30E-05 | 127.4 | 0.30 | Steady-state affinity |
-
Summary:
The fitting curves for sample-1 binding to CD32b were shown in Fig.2. Using the steady-state affinity model, the captured CD32b can bind sample-1 with an affinity constant of 1.30 x 10-5 M.
Advantages of Our FcγRIIb Binding Assay Service
- Well-established state-of-the-art technology platforms
- Abundant background knowledge and experience in antibody functional analysis
- Ph.D. level scientists and professional technical teams
- One-stop and high-quality solutions
Frequently Asked Questions
Q1: Can you assess the impact of glycosylation on FcγRIIb binding?
A1: Our analytical platform is engineered to characterize Fc domain glycosylation profiles and their effects on FcγRIIb binding interactions, including affinity and kinetic parameters. Customizable analytical workflows enable systematic investigation of these post-translational modifications as functional determinants.
Q2: What types of samples can be used for the FcγRIIb binding assay?
A2: We can handle additional pertinent biological samples, Fc-fusion proteins, and purified antibodies among other sample kinds. See our scientific team to go over the particular needs of your samples.
Q3: Are customized assay configurations feasible for FcγRIIb binding analyses?
A3: Our platform supports adaptable experimental design, with specialized protocols developed to meet specific FcγR binding study requirements. We work closely with researchers to engineer bespoke assay systems aligned with their scientific objectives, ensuring methodological rigor while addressing diverse functional interrogation needs in receptor-ligand interaction studies.
Associated Services
With extensive experience and comprehensive technology platforms, Creative Biolabs is a senior expert in the antibody development industry for many years. We are able to provide a full panel of antibody Fc functional assays, which include cell-based cytotoxicity assay services like antibody-dependent cytotoxicity (ADCC), antibody dependent cellular phagocytosis (ADCP), and complement dependent cytotoxicity (CDC), as well as non-cell based FcγRs binding (FcγRIIIa, FcγRIIIb, FcγRIIa, FcγRI), FcRn, and complement protein C1q assay services.
We believe that our high-quality services will help you get through many thorny issues of Fc function analysis. Please feel free to contact us or send us an inquiry for more details!
References
- Ben Mkaddem, Sanae, Marc Benhamou, and Renato C. Monteiro. "Understanding Fc receptor involvement in inflammatory diseases: from mechanisms to new therapeutic tools." Frontiers in Immunology 10 (2019): 811.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.
