FcγRIIIb Binding Assay Service
To address the challenges in therapeutic antibody development, specifically optimizing effector functions and ensuring clinical efficacy, Creative Biolabs offers advanced FcγRIIIb Binding Assay services. We empower you to accelerate drug discovery, precisely characterize antibody-FcγRIIIb interactions, and develop highly effective and specific antibody therapeutics through our cutting-edge biophysical platforms and innovative protein engineering expertise.
FcγRIIIb Introduction
FcγRIIIb (CD16b) is a crucial Fc receptor primarily expressed on neutrophils, playing a significant role in antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC is a vital mechanism of action for many therapeutic antibodies used in cancer and autoimmune disease treatment. Understanding the precise binding affinity and kinetics between a therapeutic antibody and FcγRIIIb is paramount for predicting its in vivo efficacy and potential safety profile. Furthermore, genetic polymorphisms of FcγRIIIb (e.g., NA1 and NA2 alleles) can influence binding efficiency and, consequently, patient response to antibody therapies. Therefore, the development and application of robust FcγRIIIb binding assays are indispensable for optimizing antibody design, facilitating biosimilar development, and enhancing the clinical success of new biologic drugs.
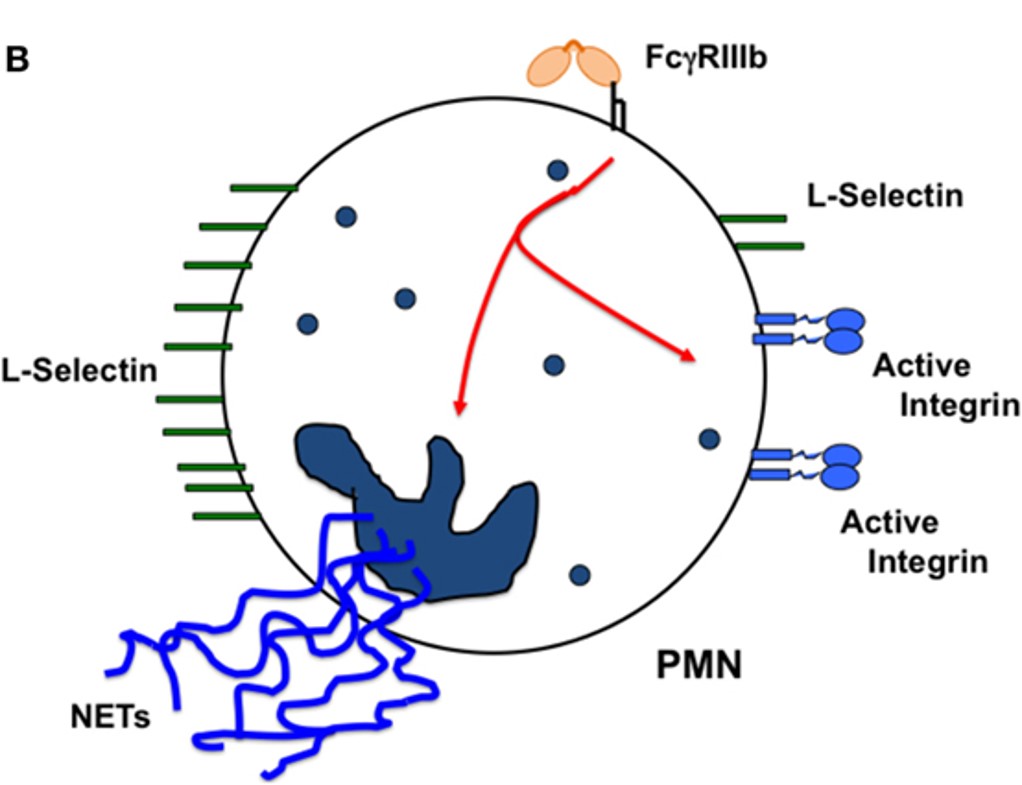 Fig.1 The cellular responses induced by FcγRIIIb.1
Fig.1 The cellular responses induced by FcγRIIIb.1
Our FcγRIIIb Binding Assay
Navigating the complexities of therapeutic antibody development often involves overcoming hurdles in understanding precise effector mechanisms and ensuring clinical relevance. Creative Biolabs provides a comprehensive FcγRIIIb binding assay to address these challenges. Our purpose is to deliver precise, quantitative data on antibody-receptor interactions, empowering you to effectively characterize therapeutic antibody candidates, evaluate biosimilar comparability, and gain critical insights into ADCC mechanisms.
We meticulously adhere to established scientific principles, employing biophysical methods like SPR and BLI, alongside immunoassay-based (ELISA) and cell-based (FACS) approaches to measure affinity and kinetics. Our process spans from initial consultation and customized assay design to high-quality recombinant protein preparation, precise binding reaction execution, sophisticated data acquisition and analysis, and culminates in a comprehensive final report. Projects typically conclude within 4 to 8 weeks, depending on specific requirements. We ensure the highest quality results through rigorous experimental design, state-of-the-art platforms, and expert analysis, providing you with actionable data for informed decision-making and optimal therapeutic development.
Workflow
Step1. Project Consultation & Design: Our scientists discuss your project goals, antibody characteristics, and requirements to tailor an optimal assay strategy. We select the most appropriate platform(s) (SPR, BLI, ELISA, or cell-based FACS) to align with your scientific objectives.
Step2. Recombinant Protein Preparation: We prepare high-quality, functional recombinant FcγRIIIb protein in-house, including NA1 and NA2 variants, ensuring relevant and accurate binding data.
Step3. Binding Reaction Execution: The FcγRIIIb or test antibody is immobilized. We then introduce the binding partner at varying concentrations, enabling real-time detection and measurement of interaction events.
Step4. Data Acquisition & Analysis: High-resolution binding data is collected. Our bioinformatics experts perform sophisticated analysis to accurately calculate key kinetic and affinity constants.
Step5. Comprehensive Reporting: All raw data, processed results, calculated parameters, and expert interpretations are compiled into a clear, detailed report, including methodology and recommendations.
Selective Approaches at Creative Biolabs
We offer a series of selective approaches to design for customers' projects, including but not limited to:
- ELISA
- Flow cytometry
- Surface plasmon resonance / Bio-layer interferometry: real-time, label-free full kinetic analysis.
Service Highlights
- Precision: Delivers accurate and reproducible quantitative data on antibody-FcγRIIIb interactions.
- Versatility: Utilizes multiple advanced platforms (SPR, BLI, ELISA, FACS) to suit diverse project needs.
- Depth: Provides detailed kinetic and affinity constants for comprehensive characterization.
- Customization: Offers specific FcγRIIIb polymorphic variants (NA1/NA2) for clinically relevant insights.
- Efficiency: Accelerates lead candidate selection and optimization, reducing development timelines.
Case Study
| Objective: | To measure the binding affinity of sample-1 with FcγRIIIb/CD16b subtypes via the SPR method. | |||||||||||||||||||||||||||||||||
| Assay Format: |
Binding and fitting curves between the sample with FcγRIIIb/CD16b (NA1): steady-state model; Binding and fitting curves between samples with FcγRIIIb/CD16b (NA2): steady-state model; |
|||||||||||||||||||||||||||||||||
| Results: |
Sample-1 binding to CD16b (NA1) The fitting curves for Sample-1 binding to CD16b (NA1) are shown in Fig.2. Using the steady-state affinity model, the captured CD16b (NA1) can bind Sample-1 with an affinity constant of 3.14×10-6 M. Sample-1 binding to CD16b (NA2) The fitting curves for Sample-1 binding to CD16b (NA2) are shown in Fig.3. Using the steady-state affinity model, the captured CD16b (NA2) can bind Sample-1 with an affinity constant of 1.99×10-6 M. |
|||||||||||||||||||||||||||||||||
|
Table 1 SPR result summary between sample-1 with FcγRIIIb/CD16b subtypes. |
||||||||||||||||||||||||||||||||||
Customer Reviews
- Exceptional Kinetic Data 2 months ago, Dr. S***h L
Using Creative Biolabs' FcγRIIIb Binding Assay in our research has significantly improved our ability to obtain high-resolution kinetic data, allowing us to differentiate subtle binding differences between antibody variants that were critical for lead optimization.
- Polymorphism Insights 4 weeks ago, Prof. M***a R
Creative Biolabs' service provided invaluable insights into our antibody's binding to FcγRIIIb-NA1 and NA2 variants. This directly informed our understanding of potential patient responsiveness and guided our clinical trial design.
FAQs
-
Q1: What types of antibodies can be tested in the FcγRIIIb Binding Assay?
A1: We can accommodate a wide range of IgG antibodies, including monoclonal antibodies, bispecific antibodies, and antibody fragments, provided they contain an Fc region capable of binding FcγRIIIb. Our team can also work with you to optimize conditions for unique antibody formats.
-
Q2: Can you help us understand the impact of FcγRIIIb polymorphisms on our antibody's efficacy?
A2: Absolutely. We routinely offer assays utilizing both FcγRIIIb-NA1 and FcγRIIIb-NA2 variants. This allows for direct comparison of your antibody's binding across these critical polymorphisms, providing crucial insights into potential patient-specific responses and helping you strategize for clinical development.
-
Q3: What kind of data will I receive from the assay, and how can I interpret it?
A3: You will receive a comprehensive report including raw data, calculated binding affinity and kinetic constants, and detailed graphs. Our expert scientists will provide an in-depth interpretation of the results and discuss their implications for your antibody's therapeutic potential and future development.
Related Sections
To further support your therapeutic antibody development, Creative Biolabs offers a range of complementary services:
- ADCC Assay: Directly measure the functional outcome of FcγRIIIb binding.
- Other Fc Receptor Binding Assays: Comprehensive analysis of your antibody's interaction with other human Fcγ receptors (e.g., FcγRI, FcγRIIa, FcγRIIb, FcγRIIIa), FcRn, and complement protein C1q to understand a full spectrum of effector functions.
Work with Creative Biolabs
Creative Biolabs is your trusted partner for advanced FcγRIIIb Binding Assay services, delivering the precise insights you need to develop highly effective therapeutic antibodies. Our commitment to scientific excellence and customer success ensures your projects are supported by the best in the industry. If you are interested in testing your therapeutics through our FcγRIIIb binding assay, we encourage global customers to contact us immediately.
Reference
- Rosales, Carlos. "Fcγ Receptor Heterogeneity in Leukocyte Functional Responses." Frontiers in immunology vol. 8 280. 20 Mar. 2017, doi:10.3389/fimmu.2017.00280. Distributed under an Open Access License CC BY 4.0. The original image was modified by extracting and using part B, and the title was changed to "The cellular responses induced by FcγRIIIb".
For Research Use Only.

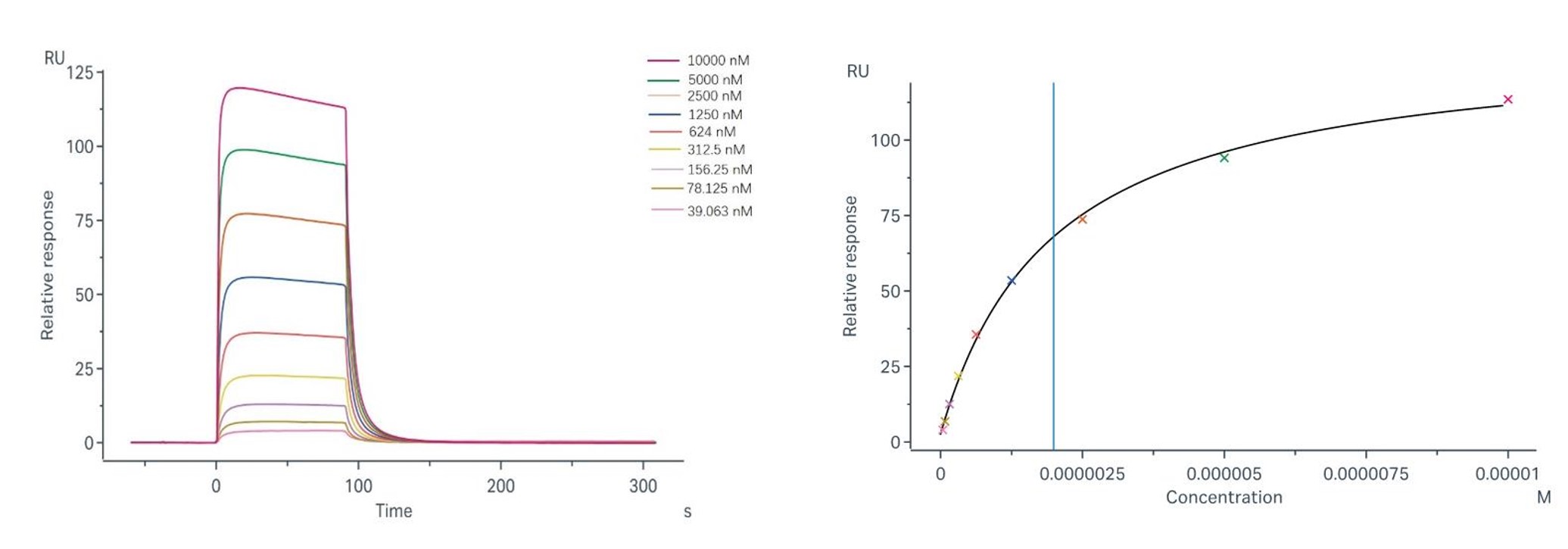 Fig.2 Binding and fitting curves between sample-1 with FcγRIIIb/CD16b (NA1) at different concentrations.
Fig.2 Binding and fitting curves between sample-1 with FcγRIIIb/CD16b (NA1) at different concentrations.
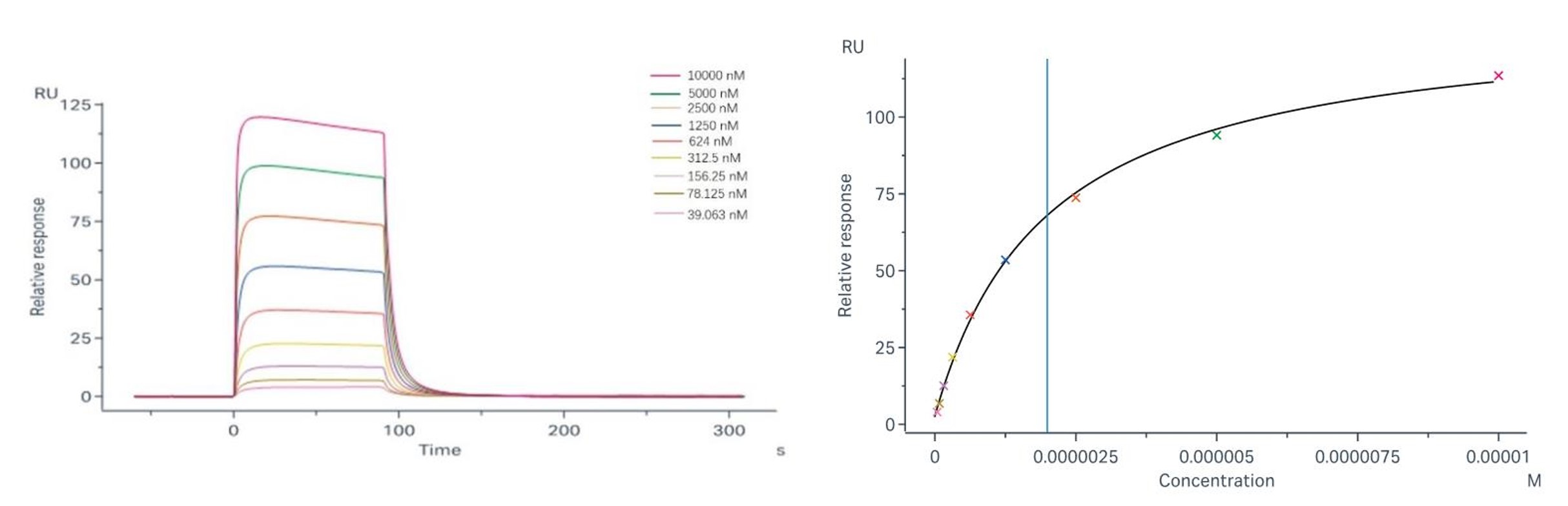 Fig.3 Binding and fitting curves between sample-1 with FcγRIIIb/CD16b (NA2) at different concentrations.
Fig.3 Binding and fitting curves between sample-1 with FcγRIIIb/CD16b (NA2) at different concentrations.